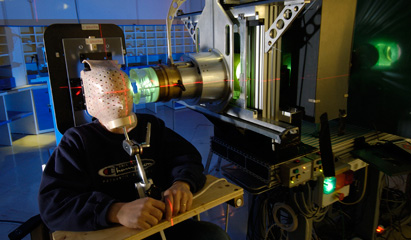
Proton therapy is an emerging dose-escalated modality of radiation therapy cancer control, involving the bombardment of tumor tissue with charged particles. Proton therapy offers a more precisely localized radiation dose than is possible with external beam radiation with x-rays or gamma rays. The result is less irradiation of nontarget tissues and reduced patient morbidity.
Although it was first proposed in 1946, proton therapy is a relatively recent innovation in terms of clinical availability, approved by the FDA in 2001. As a result primarily of cost—up to $225 million per facility1—the clinical availability of proton therapy is limited. Patient demand has driven growth, with eight cancer centers in the United States currently offering proton particle beam therapy for cancer, in Loma Linda and San Francisco, California; Indiana; Massachusetts; Texas; Florida; Oklahoma; and most recently, at the Roberts Proton Therapy Center in Philadelphia, Pennsylvania, which opened in January 2010.1-3 Several more U.S. proton beam oncology facilities are in development.1
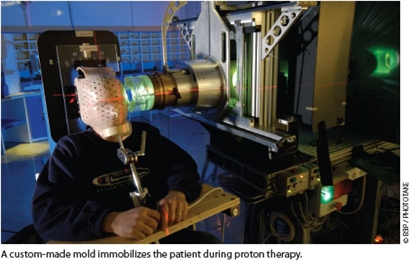 While empiric evidence supports this modality’s clinical benefits, the relative costs and benefits of proton therapy compared to traditional radiotherapies remain unclear and controversial. Debate centers on the high cost of proton therapy facility construction and the equivocal evidence for superior tumor control and patient survival compared to traditional therapies. Most published proton beam therapy studies are retrospective single-group studies, reflecting the relatively small number of U.S. facilities and the relatively short time during which the modality has been clinically available. One recent systematic review of particle beam therapies found that of 243 published papers, only 8 were randomized clinical trials comparing treatment with or without charged particles—none of which reported significant differences in cancer-specific survival rates or adverse events.1 However, these studies involved only a few thousand patients, and published particle beam studies did not utilize advanced statistical analyses, complicating interpretation of these early results.1
While empiric evidence supports this modality’s clinical benefits, the relative costs and benefits of proton therapy compared to traditional radiotherapies remain unclear and controversial. Debate centers on the high cost of proton therapy facility construction and the equivocal evidence for superior tumor control and patient survival compared to traditional therapies. Most published proton beam therapy studies are retrospective single-group studies, reflecting the relatively small number of U.S. facilities and the relatively short time during which the modality has been clinically available. One recent systematic review of particle beam therapies found that of 243 published papers, only 8 were randomized clinical trials comparing treatment with or without charged particles—none of which reported significant differences in cancer-specific survival rates or adverse events.1 However, these studies involved only a few thousand patients, and published particle beam studies did not utilize advanced statistical analyses, complicating interpretation of these early results.1
IMPROVED TUMOR TARGETING
Imprecise radiation beam targeting of tumors is the primary limitation in the use of radiotherapy for cancer treatment. Radiation therapy, usually involving electron accelerator-produced high-energy x-rays, is a mainstay for tumor control, with most cancer patients receiving radiation as part of their treatment regimen. But irradiation of nontarget tissue and resulting patient morbidity remain limiting factors in its use. Were it possible to target only tumor tissue, radiation therapy would revolutionize cancer care, offering very high control and cure rates without harm to the patient. Unfortunately, scatter, imprecise targeting, and variations in patient and tumor anatomy and movement make irradiation of nontarget tissue inevitable in clinical practice. Irradiation is effective for tumor control despite the limiting effects of incidental healthy tissue irradiation because tumor cells are dividing at more rapid rate than healthy cells and hence are usually more vulnerable to irradiation’s damaging effects on DNA than are healthy cells.
Electron accelerator-produced x-rays remain the most widely used modality for curative and palliative cancer management today. Gamma ray radiotherapy is also in widespread use. The success of tumor control depends on the total radiation dose, with doses fractionated over a period of several weeks to minimize nontarget tissue doses. While most nontarget radiation is delivered to the skin surface, x-ray and gamma ray absorption by nontarget tissues also causes exponential decreases in dose with increasing tumor depth from the body surface.4 In addition to intrinsic variations in nontumor tissue irradiation, there exists significant variation in patients’ sensitivity to radiation toxicity, which cannot be well predicted prior to irradiation.4
Protons’ large mass prevents significant beam broadening and scatter, allowing little side scatter into nontarget tissues from particle beams and superior beam focus on solid tumor tissue. Proton therapy therefore causes much lower levels of in-beam absorption of radiation by nontarget tissues, reducing morbidity and improving tumor control. Protons deliver relatively little radiation dose to the skin and instead release most of their energy when they come to rest, creating a dose curve known as the Bragg peak.4 Tissues behind or below the target tissue do not receive radiation.
Proton-beam Bragg peaks are narrow, requiring the combination of different energy-spectrum beams, collectively known as spread-out Bragg peak (SOBP), to treat an entire target tumor volume.4 SOBP represents the sum of several individual Bragg peaks at different depths. Tumors near the body surface can be treated with lower-energy protons, but because protons have narrow ranges (and resulting Bragg peaks), proton accelerators are used to produce beams of increasing energies to accommodate tumor depth.
Improved targeting allows reduced morbidity and, hence, completed radiation regimens for a larger proportion of patients. But that is not the only potential advantage of proton therapy. With increased survival times and longer life spans, particularly for younger patients, minimizing the long-term impact of nontarget tissue irradiation is increasingly important in efforts to avoid secondary tumors. (Long latency periods for most radiation-induced cancers mean that younger patients will be more likely than older patients to develop those tumors.) While the reduced scatter and nontarget tissue irradiation of proton beam therapy would seem likely to lower the risk of secondary cancers (making it a particularly attractive modality for treating childhood solid tumors, for example), empiric evidence is limited for the relative risk of secondary cancers from proton therapy.4
APPLICATIONS FOR PROTON THERAPY
The benefits of proton therapy are clearest for tumors close to the surface and near particularly radiosensitive nontarget tissues. The treatment offers superior local tumor control rates compared to traditional radiation therapies for melanoma of the eye, for example, and represents a viable alternative to surgery for this type of tumor.4 Eye melanomas and skull-base cancers, relatively rare malignancies, were also the first tumors treated with proton beam therapy; more recently, tumors of the head and neck, liver, lung, brain, breast, prostate, and pelvis have been treated with protons.1,4
The great promise of proton therapy is that reduced morbidity will allow more aggressive, dose-escalated radiation treatment for solid tumors. Systematic reviews of published peer-reviewed studies have been discouraging, however, suggesting little advantage over traditional radiotherapies for patient survival rates, tumor control, and toxicity—even for those tumors, such as eye melanoma, for which particle beam treatments seem best suited.1,5
X-rays stimulate tumor angiogenesis (recruitment of new blood vasculature), increasing the risk of recurrence and metastasis.4 Carbon particle beam irradiation destroys capillary cell growth in vitro and in vitro human lung cancer cell invasion of tissue, suggesting that particle therapies, including proton therapy, may inhibit angiogenesis and tumor spread.4 However, clinical studies (and even in vitro studies, in the case of proton therapy) of particle beam treatment’s effects on angiogenesis and metastasis in cancer patients have yet to be reported.4
The current empiric case for proton therapy’s advantages over other radiotherapies is not encouraging. But as the number of cancer patients whose treatments include proton beam radiation grows, better data sets will become available for statistical assessment of this modality’s relative strengths and weaknesses compared with traditional radiotherapies. If results are favorable, the availability of proton therapy will almost certainly expand despite the high initial (construction) costs.
CLINICAL TRIALS
Very few randomized clinical trials have compared proton beam therapy with conventional x-ray radiotherapy, and systematic reviews of the literature agree that there is currently not enough evidence to support the cost effectiveness or clinical superiority of proton beam therapies for cancer treatment.5,6 Nevertheless, some authors have argued that the underlying logic—depth/dose distribution characteristics and tumor-to-healthy tissue irradiation ratios—and some nonrandomized trial data, taken together, are so compelling that it is “hard to … avoid the conclusion that there is … a high probability that protons can provide superior therapy to that possible with x-rays in almost all circumstances” and that it is “ethically unacceptable to conduct randomized clinical trials comparing protons with x-rays” because such trials would deny some patients access to proton therapy.7 This minority view is flatly rejected by other authors, who have argued, for example, that “any complex and expensive technology, including proton therapy, should not be employed on the basis of belief alone….”5
Current clinical trials of proton radiotherapy for cancer are limited to early (phase I and II) trials. A search of the National Cancer Institute’s clinical trials database (www.cancer.gov/clinicaltrials/search, accessed June 9, 2010) for the phrase proton beam identified 9 phase I trials and 32 phase II trials. No phase III or IV trials were identified. Actively-recruiting trials include studies of pineoblastoma, retinoblastoma, pancreatic and hepatocellular cancers, chloangiocarcinoma, head and neck carcinomas, gliomas, oropharyngeal and nasopharyngeal cancers, non-small cell lung cancers, cervical cancer, and rhabdomyosarcoma.
As of June 9, 2010, two new clinical trials, both at the Abramson Cancer Center of the University of Pennsylvania, had begun recruiting patients within the past month: a no-phase-specified feasibility study of proton radiotherapy for re-irradiation of recurrent malignancies (protocol NCT01126476; contact John Plastaras, MD, PhD, 215-662-2812) and a feasibility/phase II study of grade I-III meningioma and hemangiopericytoma brain cancers (protocol NCT01117844; contact Robert Lustig, MD, 215-662-2812). Two active epidemiologic studies were also identified: a quality-of-life study for prostate proton therapy patients (protocol NCT00489814, no phase specified; contact Andrew K. Lee, MD, M.D. Anderson Cancer Center, Houston, TX, 713-563-2348) and a study of the late effects of proton radiation therapy in low-grade glioma patients (protocol NCT00681473, no phase specified; contact Helen A Shih, MD, MS, MPH, Massachusetts General Hospital, Boston, 617-643-3510). ONA
Bryant Furlow is a medical writer living in Albuquerque, New Mexico.
REFERENCES
1. Terasawa T, Dvorak T, Ip S, et al. Systematic review: charged-particle radiation therapy for cancer. Ann Intern Med. 2009;151(8):556-565.
2. Particle Therapy Cooperative Group. Particle therapy facilities in operation. http://ptcog.web.psi.ch/ptcentres.html. Last updated March 25, 2010. Accessed June 9, 2010.
3. Proton therapy: future directions. MedicalPhysicsWeb. http://medicalphysicsweb.org/cws/article/opinion/41256. December 16, 2009. Accessed June 9, 2010.
4. Durante M, Loeffler JS. Charged particles in radiation oncology. Nature Rev. 2010;7(1):37-43.
5. Brada M, Pijls-Johannesma M, De Ruysscher D. Current clinical evidence for proton therapy. Cancer J. 2009;15(4):319-324.
6. Piljls-Johannesma M, Grutters JPC, Verhaegen F, et al. Do we have enough evidence to implement particle therapy as standard treatment in lung cancer? A systematic literature review. Oncologist. 2010;15(1):93-103.
7. Goitein M. Should randomized clinical trials be required for proton radiotherapy? J Clin Oncol. 2008;26(2):175-176.