6 surprisingly healthy hawker food in Singapore

- 6 surprisingly healthy hawker food in Singapore to try
- 1. Mushroom and minced pork noodles are actually better than fishball noodles
- 2. Roast duck is high in iron, which boosts your focus
- 3. Stir-fried beef with ginger is good for your post-workout meal
- 4. Ikan assam pedas (assam fish) is better for your brain — and heart
- 5. Soto ayam (chicken soup) is a well-balanced meal
- 6. Wanton soup with chye sim will fill you up — even without extra noodles
Is there such a thing as healthy hawker food in Singapore or is it just an urban myth?
I’m sure we’ve all been there — it’s lunchtime and you just want to eat something more wholesome, but your colleagues want lunch at the same old hawker centre instead of a salad bar.
You could ditch for your bowl of raw veggies, but you could learn to make smarter choices at the hawker centre, such as opting for low-calorie hawker food or looking for healthy hawker food options.
Don’t worry, you don’t have to be stuck with boring ol’ fish soup.
Consider these other healthy hawker dishes — they pack fewer than 600 calories per serving, and rank among the most nutritious options available at hawker centres.
We get Jaclyn Reutens, dietitian at Aptima Nutrition and Sports Consultants, to weigh in on these surprisingly healthier hawker foods in Singapore.
6 surprisingly healthy hawker food in Singapore to try
1. Mushroom and minced pork noodles are actually better than fishball noodles
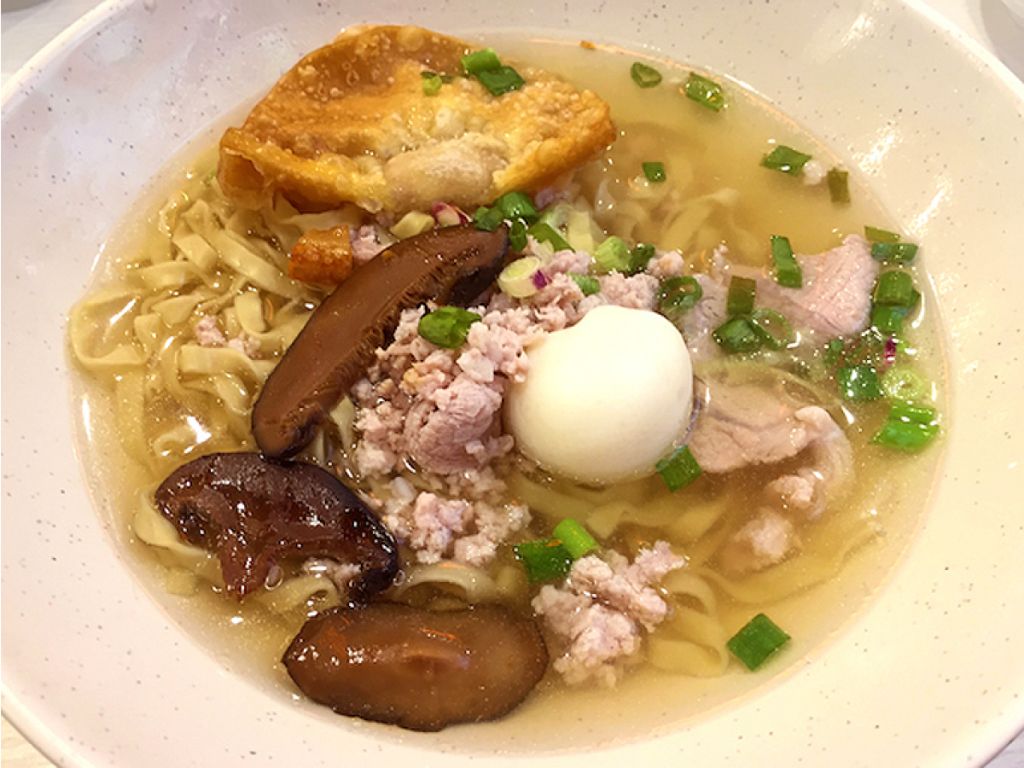
Who knew? The tastier of the two is also more nutritious. Mushroom and minced pork noodles (bak chor mee) make a more balanced meal than fishball noodles, which are higher in carbohydrates but lower in other nutrients.
Dietitian Jaclyn Reutens says: Factory-made fishballs are mainly flour-based and have very little protein. On the other hand, minced pork provides protein and zinc, nutrients that are essential for our body.
Mushrooms, which give the dish its umami punch, also pack plenty of good-for-you nutrients. These include B vitamins, which help you release energy from food, potassium, which lowers blood pressure, and selenium, an antioxidant that gets rid of harmful free radicals.
Nutrition score of mushroom and minced pork noodles in soup (594g):
381 calories, 17g protein, 12.2g fat (4.7g saturated), 8g fibre, 50g carbohydrates, 37mg cholesterol, 1732mg sodium
2. Roast duck is high in iron, which boosts your focus

When you’re at the roast meat stall, pass on the char siew (barbecued pork) and sio bak (crispy roast pork belly) in favour of roast duck. Duck has more of the good stuff, such as protein and minerals, and less of the bad, such as sugar and fat. Remember to remove the skin if you’re watching your diet.
Dietitian Jaclyn Reutens says: Compared with roast pork, duck meat is lower in fat but higher in iron. A mineral needed by both men and women alike, iron helps the body generate red blood cells. It also improves concentration levels.
Moreover, duck is packed with good-quality protein that assists in daily cell renewal. Roast duck is actually better than the braised version because the braising process retains more fat.
Nutrition score of duck rice, with skin removed (350g):
530 calories, 24g protein, 11g fat (3.6g saturated), 3g fibre, 84g carbohydrates, 80mg cholesterol, 326mg sodium
3. Stir-fried beef with ginger is good for your post-workout meal

Stir-fried beef with ginger, a popular zi char dish, is in fact one of the leanest and most protein-rich options on the menu, if you’re looking for healthy Singapore hawker food.
The star protein, beef, is also a great source of essential minerals such as iron and zinc, which makes it an ideal candidate when searching for healthy meals in Singapore.
Dietitian Jaclyn Reutens says: This savoury beef dish is better than sweet and sour pork, which is coated in flour, deep-fried and then tossed in a sugary sauce.
It is also healthier than gong bao ji (Szechuan chicken), which can be fatty as it typically features thigh meat with skin. Eating beef after a workout helps with muscle repair, too, so this makes great post-gym nosh.
Nutrition score of stir-fried beef with ginger (196g):
298 calories, 27g protein, 19g fat (8.2g saturated), 4g fibre, 4g carbohydrates, 49mg cholesterol, 1494mg sodium
4. Ikan assam pedas (assam fish) is better for your brain — and heart
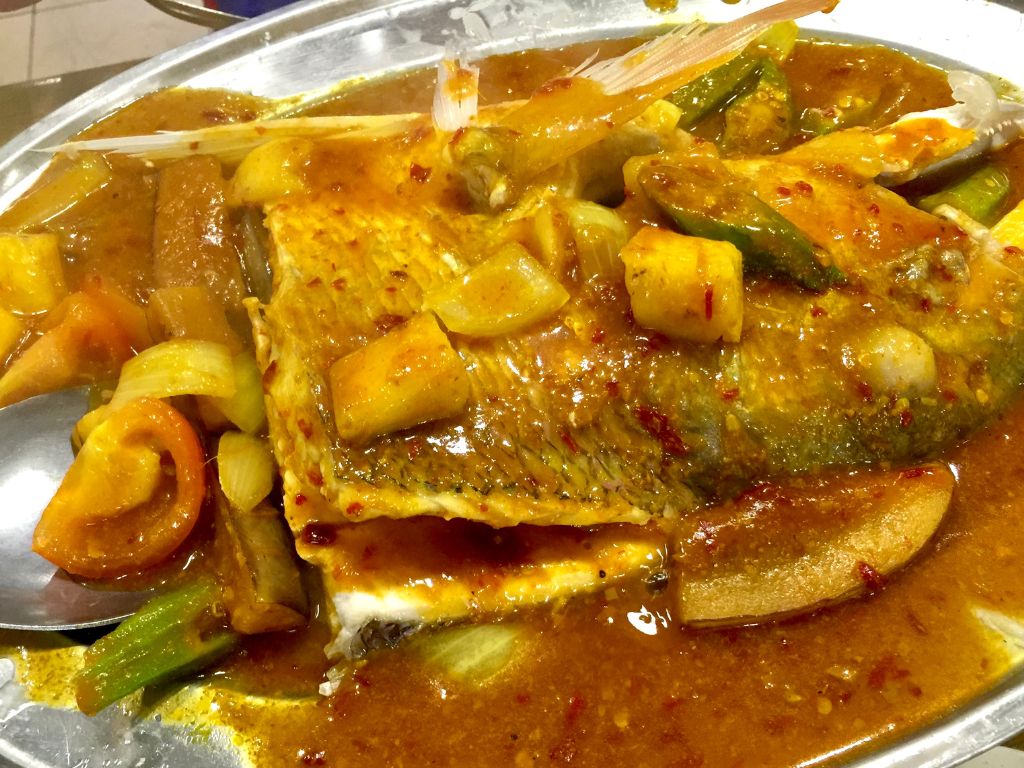
Move over, fish head curry.
Wise folks will pick assam fish over curry because it’s prepared in a healthier way — with an array of piquant spices — and doesn’t lose out on flavour.
Dietitian Jaclyn Reutens says: Fish is brain food; it’s high in omega-3 fatty acids and keeps your mood up, too! Unlike curry fish, assam fish does not contain fatty coconut milk, which is bad for your arteries.
Another plus — ikan assam pedas is often cooked with loads of vegetables, such as eggplant, lady’s finger, tomato and onions. Ask the hawker for more veggies to up your fibre intake.
Nutrition score of assam fish (184g): 123 calories, 21g protein, 3.7g fat (2.2g saturated), 2g fibre, 2g carbohydrates, 61mg cholesterol, 532mg sodium
5. Soto ayam (chicken soup) is a well-balanced meal

Feel like slurping something belly-warming?
Your choices aren’t confined to fish soup and yong tau foo. Soto ayam is a light yet satisfying healthy hawker food you can get at most Malay stalls.
Dietitian Jaclyn Reutens says: There are not many low-calorie Malay dishes, but soto ayam is one of them. It has fewer than 300 calories per serving. In contrast, other popular Malay dishes, such as mee rebus and mee siam, have more than 500 calories each. Soto ayam is a well-balanced dish as it provides carbohydrates from the ketupat (rice cubes), protein from the chicken, and fibre from the beansprouts. Sedap! (Malay for tasty)
Nutrition score of soto ayam (569g): 219 calories, 19 protein, 8g fat (2.9g saturated), 8g fibre, 18g carbohydrates, 17mg cholesterol, 2418mg sodium
6. Wanton soup with chye sim will fill you up — even without extra noodles

Comfort food doesn’t have to be cloying and fattening.
Wanton soup checks the right boxes for many of us; it’s hot, soupy, meaty and delicious! Top it off with extra chye sim (mustard greens) — most stalls offer the option at an extra dollar — and you get an added dose of vitamins and fibre!
Dietitian Jaclyn Reutens says: It may not look like a complete dish, but it is, even without the noodles. You get carbs from the wanton skin, while the minced meat filling provides you with zinc, protein and B vitamins.
Chye sim is not only rich in fibre, but potassium, too. This dish is way better than pan-fried gyoza and deep-fried wanton.
Nutrition score of wanton soup with chye sim (436g):
258 calories, 13g protein, 14g fat (5.7g saturated), 2g fibre, 20g carbohydrates, 39mg cholesterol, 1783mg sodium
Nutrition data source: Health Promotion Board of Singapore
All values are rounded to the nearest whole number, except for fat, which is rounded to one decimal place.
This is an updated version of an archived article. Li Yuling wrote the original article.







