Pain is common in cancer — one-third of patients undergoing treatment and three-fourths of those with advanced disease experience it — and pain is among the symptoms patients fear the most. “It is imperative that physicians and nurses caring for these patients be adept at the assessment and treatment of cancer pain,” say the authors of Adult Cancer Pain, a practice guideline published by the National Comprehensive Cancer Network (NCCN).
 Oncology nurses have an important role here, says Judith Paice, PhD, RN, director of the cancer pain program at the Robert H. Lurie Comprehensive Cancer Center of Northwestern University, Chicago, Illinois, and a member of the panel that issued the guideline. “Nurses see patients more often, and patients are often reluctant to report pain to their physicians because they don’t want to distract them from cancer care. Patients are more inclined to discuss pain with nurses.”
Oncology nurses have an important role here, says Judith Paice, PhD, RN, director of the cancer pain program at the Robert H. Lurie Comprehensive Cancer Center of Northwestern University, Chicago, Illinois, and a member of the panel that issued the guideline. “Nurses see patients more often, and patients are often reluctant to report pain to their physicians because they don’t want to distract them from cancer care. Patients are more inclined to discuss pain with nurses.”
In recent years, “awareness that pain management is not only for end-of-life care has been increasing,” says Dr Paice. “There is a new population of patients who have essentially been cured or who can expect long-term survival but have been left with serious pain issues as a result of treatment. They may be using pain medication for life.” Aggressive pain management is starting earlier in care, she adds, with the realization that such management can make possible effective chemotherapy or radiotherapy that might otherwise be cut short because of painful side effects like neuropathy.
COMPREHENSIVE ASSESSMENT
The first step in pain management is one that most nurses (and doctors) neglect, notes Dr Paice. “Instead of making a full assessment, as soon as they hear the patient has pain, they jump to a medication they feel comfortable with.”
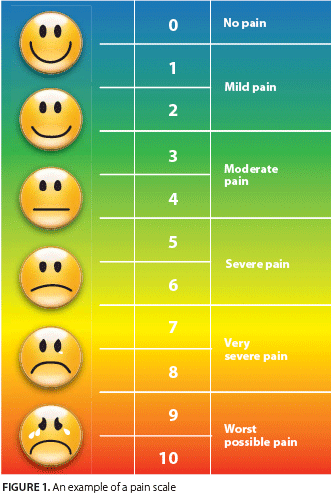
At the initial screening, ask the patient to rate the intensity of his or her pain using a numerical, categorical, or pictorial scale, and to characterize its quality (Figure 1). Determining whether pain is throbbing, aching, tingling, or sharp may provide useful information about its etiology and helps to direct treatment.
The goal of the full assessment is a “pain diagnosis” to guide an individualized treatment plan. It should include the onset and course of the pain, exacerbating and ameliorating factors, relief measures that have already been tried, and historical information relating to the patient’s overall experience with opiates and other pain management strategies. Medical and especially oncologic history (including current and prior chemotherapy, radiation, and surgery), along with medication history, provide an important context for treatment. Physical examination and laboratory findings may identify causes of pain (such as spinal cord compression) requiring specific remedies.
Psychosocial issues—family support and distress associated with pain and disease—should also be evaluated, the guideline suggests. Concern about risk of addiction has come to the fore in recent years: “Ask about the use of recreational drugs and any history of addiction or abuse—including family history,” Dr Paice emphasizes. Trouble in the past “doesn’t mean we withhold medication, but perhaps we provide a different structure and are more attentive to our prescribing patterns.”
CHOOSING AN ANALGESIC
Pain intensity is the key variable in analgesic choice for patients who are not already taking opioids.
 When the patient rates pain as severe (for example, 7 on a 10-point scale), a short-acting opioid, titrated rapidly, is the treatment of choice, augmented with other drugs if the patient has neuropathic pain.
When the patient rates pain as severe (for example, 7 on a 10-point scale), a short-acting opioid, titrated rapidly, is the treatment of choice, augmented with other drugs if the patient has neuropathic pain.
The protocol is similar for patients with more moderate pain (4-6 on a 10-point scale), except that titration of the opioid can be slower.
For pain of mild intensity (1-3), NSAIDs and acetaminophen are alternatives to opioid therapy.
Whatever the pain intensity or primary analgesic, the addition of a co-analgesic should be considered when there are indications of a cancer pain syndrome: neuropathic pain, bone pain, or pain associated with inflammation. If the pain is related to an oncologic emergency, appropriate steps (such as surgery, corticosteroids, radiotherapy, or antibiotics) must also be taken. Pain in a patient who is already taking opioids requires an adjustment of dosage, reevaluation for possible underlying causes, and perhaps the addition of co-analgesics.
OPIOID GUIDELINES
The most commonly used short-acting opioids are morphine, hydromorphone, fentanyl, and oxycodone. The guidelines advise against meperidine and propoxyphene, particularly for long-term or high-dose use, because of toxicity; they caution that partial agonists and mixed agonist-antagonist preparations are of limited utility in cancer pain and can precipitate withdrawal in opioid-dependent patients. Tramadol is weaker than other opioids but may have some value for mild to moderate pain.
Oral administration is best, as it is usually the easiest form of delivery. For patients not already taking opioids, the drug should be initiated at the equivalent of 5 mg to 15 mg of morphine sulfate orally, or 2 mg to 5 mg intravenously, and titrated to a level sufficient to relieve pain throughout the dosing interval without causing intolerable adverse effects. For breakthrough pain during opioid therapy, administer 10% to 20% of the 24-hour dose orally, or 10% intravenously.
Efficacy and side effects should be reassessed every 60 minutes (15 minutes after intravenous administration). If moderate to severe pain remains unchanged or has increased, raise the dosage by 50% to 100%; if severe pain has become moderate, repeat the same dose and reassess an hour later. When pain has been relieved or reduced to mild levels, consider converting to an extended-release agent or a non-opioid analgesic, providing short-acting “rescue medication” as needed.
Managing side effects is a key component of opioid therapy. Constipation is a problem best prevented by a bowel regimen, initiated along with the drug, that includes a stimulant laxative and stool softener. If constipation develops, increase the stool softener-laxative (after ruling out bowel obstruction), or consider reducing the opioid dosage and adding a nonopioid analgesic.
Nausea may respond to prochlorperazine, haloperidol, or metoclopramide. Consider an antiemetic prophylactically for a patient who has a history of opioid-induced nausea. Pruritus is best treated with an antihistamine.
Motor and cognitive impairment usually resolve spontaneously within 2 weeks of stable opioid administration. Sedation may require a dose reduction, more frequent administration to reduce peak concentration, or the addition of caffeine, a psychostimulant, or modafinil. Respiratory depression and acute changes in mental status, although rare, can be addressed immediately with a reversing agent such as naloxone.
The efficacy/side-effect ratio for the individual patient may differ among drugs, so opioid rotation—switching to an equivalently dosed different agent—is an option when side effects are difficult to control at therapeutically necessary levels.
