Abstract: The majority of patients with castrate-resistant prostate cancer will have metastatic disease at the time of diagnosis. Investigative efforts on new therapeutics for this patient population have improved with the development of androgen signaling inhibitors, such as abiraterone and enzalutamide, and PARP inhibitors, such as rucaparib and olaparib, to accompany the previously FDA-approved docetaxel, cabazitaxel, sipuleucel-T, and Radium 223. However, new therapeutic strategies are necessary to prolong survival as progression after these agents is inevitable. CDK4/6 inhibitors have advanced the field of estrogen receptor positive breast cancer treatment and are being investigated in prostate cancer given the role of androgen receptor signaling effects on the cell cycle. Response to CDK4/6 inhibitors may be predicted by the tumors’ genomic profile and may provide insight into combinatory therapy with CDK4/6 inhibitors in order to delay resistance or provide synergistic effects. Here, we review the use of CDK4/6 inhibitors in prostate cancer and potential combinations based on known resistance mechanisms to CDK4/6 inhibitors, prostate cancer regulatory pathways, and prostate-cancer-specific genomic alterations.
Keywords: metastatic castrate-resistant prostate cancer, CDK4/6 inhibitors, genomics, combination therapy
INTRODUCTION
In 1853, surgeon J. Adams once described prostate cancer (PCa) as a very rare disease. Fast forward over 160 years, PCa is now the second leading cause of cancer deaths among men in the United States.1 Despite androgen deprivation therapy (ADT), PCa will eventually develop resistance known as castrate-resistant prostate cancer (CRPC) and thus, progress to end-stage disease. There are currently eight FDA-approved therapies indicated for metastatic castrate-resistant prostate cancer (mCRPC) that include: docetaxel, abiraterone, enzalutamide, cabazitaxel, sipuleucel-T, radium-223, rucaparib, and olaparib. Unfortunately, the overall survival benefit from these therapies ranges from 2.4 to 4.8 months, demonstrating the importance of continued development of new therapeutics.2
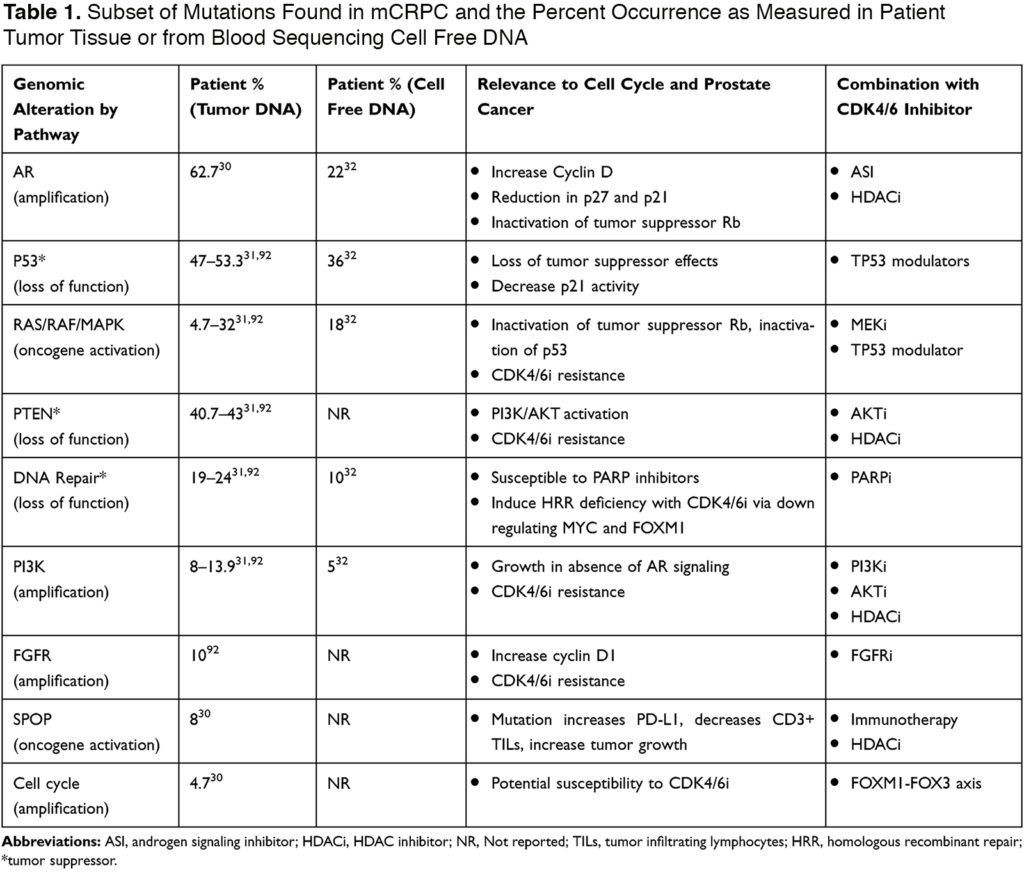
The cell cycle contains key regulatory factors to control cellular growth. These factors include enzymes such as cyclin-dependent kinases (CDK).3 In cancer, this pathway is universally disrupted resulting in tumorigenesis and over the past decade has become a focus of cancer therapeutics leading to the development of CDK4/6 inhibitors (CDK4/6i).4 CDK4/6i’s first gained approval in 2015 when palbociclib showed encouraging activity in breast cancer with clinical trials showing a double in median progression-free survival (mPFS) and also an overall survival (OS) benefit. Estrogen receptors, as well as androgen receptors, are key regulators of the cell cycle and instrumental in the regulation of transcription genes that allow for G1 to S transition.5,6 In PCa, this hormone pathway can be targeted with ADT; however, PCa eventually becomes androgen resistant which provides opportunity for the use of CDK4/6i’s to disrupt AR signaling. In patients with hormone sensitive prostate cancer (HSPC), a Phase II clinical trial (NCT02059213) investigating ADT ± palbociclib showed tolerability and multiple other trials are underway with combining androgen signaling inhibitors with CDK4/6i’s. Understanding the cell cycle as well as associated regulatory pathways and PCa specific genomic mutations (Table 1) will provide insight to potential combinatorial therapies with CDK4/6i’s. Table 1 shows mutated genes in mCRPC and will be referred throughout this review in guiding novel combinatorial therapeutic strategies.
READ FULL ARTICLE
![]() From DovePress
From DovePress
