RESULTS
Metastatic disease in head and neck cancer
The combination of cetuximab with chemotherapy has traditionally been considered standard treatment for these patients.4 However, in patients that progress after chemotherapy or in frail patients, RT has been used as a palliative treatment, with a high rate of symptom palliation and an improvement in quality of life.
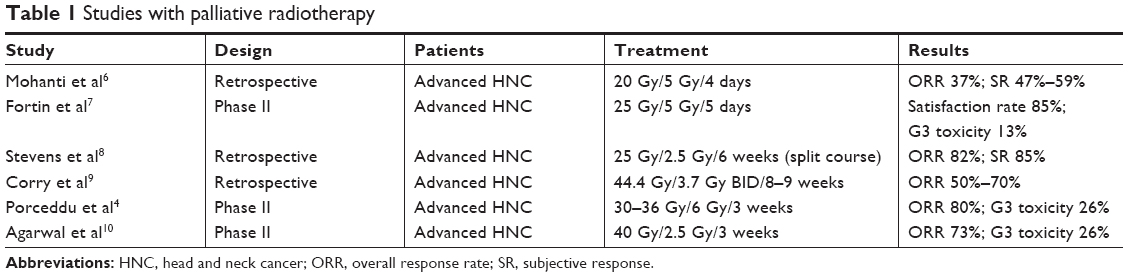
There is no consensus on the most appropriate treatment scheme. To increase patient comfort, reducing the number of hospital visits and shorter regimens using low biological doses have been tested. Mohanti et al (AIIMS study) proposed a five-fraction course of treatment to a total dose of up to 20 Gy, showing modest tumor response (37%) and symptom relief (47%–59%).6 Fortin et al recently performed a phase II study with a dose of 25 Gy in five fractions administered with intensity-modulated RT. Although treatment response was not reported, self-reported outcomes showed that 85% of patients were satisfied with the treatment, and it had a toxicity as low as 13% grade III toxicity, with no grade IV–V toxicity reported.7
The association between a higher biological dose and an increase in tumor response and survival shown in different studies8 has led to the investigation of longer RT schemes with higher doses of radiation (Table 1). Stevens et al performed a retrospective multivariate analysis in a cohort of patients treated with several fractional regimens. One of the most commonly used was a split course composed of two cycles of 25 Gy in ten fractions given within 2 weeks, separated by a 2-week break to a total dose of 50 Gy. Treatment response was 82%, while 85% of patients had an improvement in their symptoms.8 Corry et al published the results of “The Quad Shot regimen”, that consisted of three courses of twice-daily 3.7 Gy fractions for two consecutive days, completing a total dose of 44.4 Gy in twelve fractions over 8–9 weeks. An overall palliation rate of 80% was reported, with a tumor-response rate of 50%–70%.9 Porceddu et al used higher doses per fraction, delivering 30–36 Gy in five to six twice-weekly fractions of 6 Gy. An objective response of 80% was achieved, and 62% patients reported an overall improvement in quality of life. Treatment tolerance was high, with 88% of patients receiving ≥30 Gy. However, 26% of patients experienced G3 mucositis, and 11% reported severe dysphagia.4 Finally, Agarwal et al described a treatment response of 73% and >75% symptomatic relief with a palliative RT-alone regimen delivering 40 Gy in 16 fractions. It should be noted that G3 mucositis was described in 26% of the patients, and 11% required nutritional support with a gastric tube.10
(To view a larger version of Table 1, click here.)
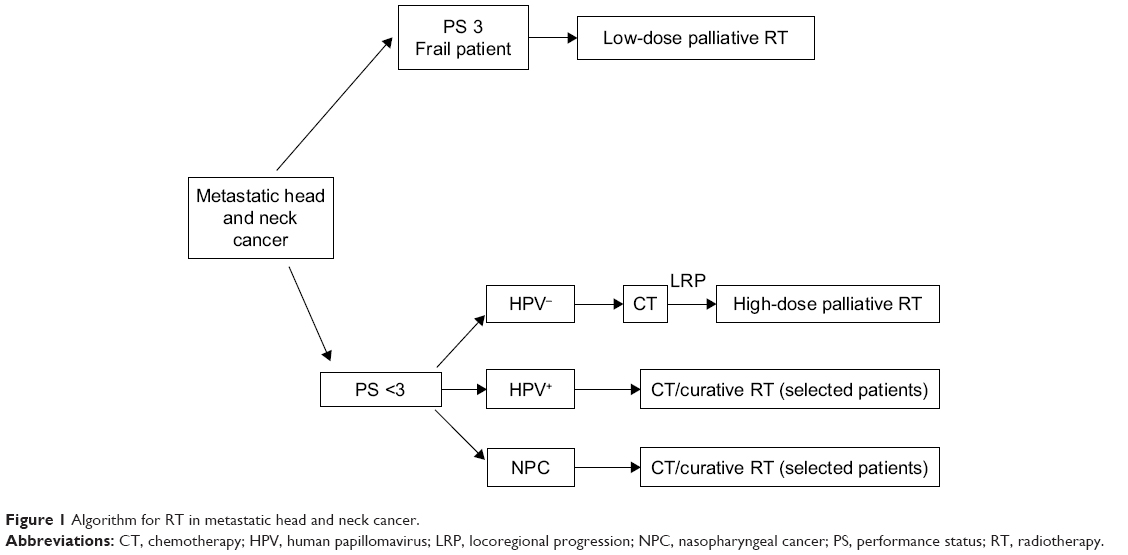
Therefore, in patients with better performance status (PS; 0–2), a palliative regimen using a higher biological dose may be indicated, provided that when selecting patients consideration is given to the fact that a significant number may experiment high mucosal toxicity (> G2). On the other hand, among patients with suboptimal PS (>2) or short survival expectancy, a palliative regimen with a low dose is indicated. An algorithm for RT in mHNC is described in Figure 1.
(To view a larger version of Figure 1, click here.)
Oligometastatic disease in head and neck cancer
Oligometastatic disease is a term coined by Hellman and Weichselbaum11 that describes a less advanced state of metastatic disease that may produce a limited number of metastases over long periods of time, amenable to potential curable local therapy.12 Data point to the hypothesis that development of widespread metastases may occur because of seeding from oligometastasis after more alterations in the chromosome have been accumulated. In HNC, oligometastatic disease may present different prognoses, depending on the location, status of the primary tumor location, and tumor histology.
Patients with primary tumors controlled
It has been shown that a subset of patients with mHNC and favorable prognostic factors may present long survival. A recent systematic review has shown that in patients with a controlled primary tumor, metastasectomy for metachronous pulmonary metastasis may offer prolonged survival for selected patients, with an overall absolute 5-year survival rate of 29.1%.13 Moreover, different retrospective studies of selected patients with surgically treated oligometastatic disease in the lung have shown 5-year survival rates of 30%–60%.14 To select which patients might benefit from this approach, different studies have shown that the presence of cervical metastases on diagnosis of the primary tumor,15tumors located in the oral cavity,16 incomplete pulmonary resection,15 and the presence of multiple pulmonary nodules17 significantly decrease the survival-rate probability for oligometastatic disease.
Stereotactic body RT (SBRT) consists in the delivery of a high ablative dose through the application of one to five high-dose fractions.18 A high dose per fraction will deliver a higher biological effect in normal tissue with slow proliferative capacity (low α:β ratio) compared with most tumors or rapid proliferative capacity tissue (high α:β ratio).19 However, the advances in techniques and imaging accomplished in recent years have contributed to the design of treatments with higher conformation and improvement in RT precision, with reproducible immobilization, precise target localization, and tumor tracking, all of which make it possible to treat tumors with high ablative doses and low toxicity.
SBRT has been successfully tested in lung cancer and metastatic disease in the lung or liver, where the parallel structure of the lung or liver allows delivery of a high dose to a minimum volume of tissue without clinical manifestations.20 The use of SBRT for the treatment of metastasis derives from excellent results obtained in early lung cancer, improving the suboptimal results obtained in the past with external RT, in which the amount of lung tissue irradiated did not allow ablative doses of radiation to be reached.
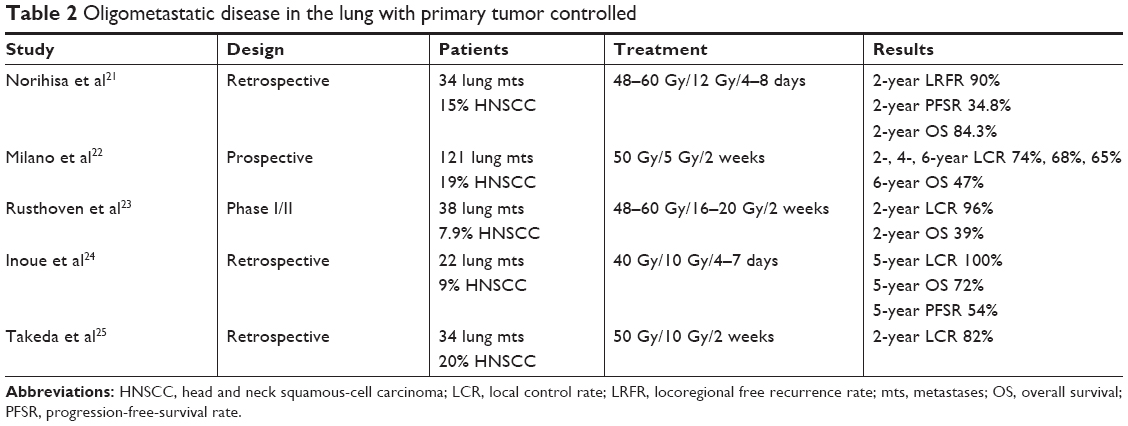
Different nonrandomized studies have shown that SBRT is a safe and effective method to treat lung metastases derived from different tumors, obtaining similar results to surgery. Local control rates of 80%–85% have been described,21–24 as have 2- to 3-year progression-free survival of 20% and a predominant pattern of recurrence at a distance.23 However, most of these series did not focus exclusively on HNC, and presented a variety of metastases of different origins with a low percentage of HN tumors that ranged between 8%23 and 18%24 (Table 2). Moreover, although some authors have described better control of metastasis in HNC compared with metastasis from colorectal cancer,25 it is not possible to reach definitive conclusions, due to the low number of HN tumors included in the series analyzed. Similar results have been observed with liver metastasis. Katz et al reported the results of 174 liver metastases from different tumors. With a median of 15 months, local control was 76% and 57% at 10 and 20 months, respectively. No grade III toxicity was reported.26
(To view a larger version of Table 2, click here.)