Uterine cervical cancer is the second most common cancer diagnosis among women worldwide, after breast cancer. Most cases are caused by infection with human papillomavirus (HPV). Mortality is higher in Africa and parts of Asia because diagnosis is more frequently made at later tumor stages. In the United States, locally advanced and metastatic cervical cancers are relatively rare but can represent a daunting treatment challenge. Five-year survival rates decline sharply with later disease stage at diagnosis, from 91% for early-stage localized tumors to 58% for disease involving regional lymph nodes and only 17% for metastasis to distant organs.1
Tumor size and stage at diagnosis are prognostic indicators; women with large stage I tumors (more than 4 cm in diameter) and women with stage II or more advanced cancers experience high treatment-failure rates and shorter survival times.2
The overall age-adjusted US death rate is approximately 2.4 per 100,000 women annually, based on mortality from 2003 to 2007.1 Death rates vary markedly by ethnicity, however. Mortality rates among blacks, Native Americans, Hispanics, and whites are 4.4, 3.4, 3.1, and 2.2 per 100,000 women, respectively.2,3 Overall, cervical cancer mortality rates declined sharply in the United States between 1975 and 1982, and between 1996 and 2003, likely reflecting intensified screening efforts during those years.3 Screening and early detection among rural and Indian reservation populations remains relatively poor.
The downward trend in cervical cancer mortality has waned since 2003.1,3 Researchers are hopeful that widespread vaccination against HPV among young sexually active women will reduce cervical cancer incidence.
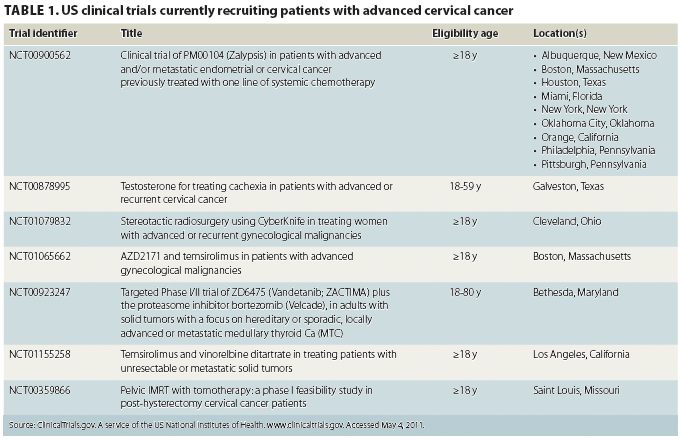
Recruitment of sufficient numbers of participants is challenging for US researchers conducting clinical trials of novel cervical cancer treatments; therefore, the pool of research data from which evidence-based recommendations and guidelines can be drawn is limited. Table 1 lists clinical trials for cervical cancer treatment that are actively recruiting participants. Only a few meta-analyses of cervical cancer clinical-trial data have been conducted.
 “Some proposed studies have not been authorized by the National Cancer Institute cervical task force for fear they would not accrue [enough volunteers] or are competing with ongoing trials,” explained Claire Verschraegen, MD, professor of medicine at the University of New Mexico Cancer Center. “Obviously, developing countries are the place to run cervical cancer trials.”
“Some proposed studies have not been authorized by the National Cancer Institute cervical task force for fear they would not accrue [enough volunteers] or are competing with ongoing trials,” explained Claire Verschraegen, MD, professor of medicine at the University of New Mexico Cancer Center. “Obviously, developing countries are the place to run cervical cancer trials.”
TREATMENT STRATEGIES
Concurrent chemoradiation therapy (CCRT) Radiation plays a critical role in treatment of locally advanced cervical cancer. Radiation is not recommended as a component of treatment only when the patient has a history of pelvic radiation, or in the rare cases in which the patient refuses radiotherapy, Verschraegen told Oncology Nurse Advisor (ONA).
External-beam radiotherapy is used to sterilize the pelvic lymph nodes and to reduce tumor diameter. Radiation-bead brachytherapy is then used to assault the primary tumor.
Radiation alone controls locally advanced cervical tumors (stage IB) in fewer than 65% of cases. Combined with platinum-based chemotherapy agents, however, patient outcomes are better—tumor control is achieved in up to 75% of cases.2 Concurrent chemoradiotherapy achieves greater local tumor control and lower relapse rates.
The National Cancer Institute (NCI) 1999 consensus statement, which is supported by subsequent meta-analyses that pooled data from available clinical trials, recommends concurrent chemoradiation with platinum-based agents (usually cisplatin [Platinol, generics]) rather than radiotherapy alone.2,4-7 The recommended regimen in the NCI consensus statement is intravenous cisplatin 40 mg/m2 weekly for 6 weeks or two cycles of cisplatin (40 mg/m2) plus 5-fluorouracil (Adrucil, generics) (1 g/m2 as 96-hour infusions) concurrent with radiation on days 1 to 5 and days 22 to 26 of radiotherapy;2 the former is the current most commonly used regimen. The number of cisplatin cycles and brachytherapy use appear to improve survival rates. Patients who receive fewer than six delivered cycles or do not receive brachytherapy experience reduced progression-free survival and reduced overall survival.8
 Alternative treatment strategies Two controversial alternative neoadjuvant chemotherapy strategies may be used, sometimes in an effort to conserve fertility in younger patients. One strategy involves chemotherapy followed by surgery or radiotherapy, relying on the chemotherapy agents to reduce tumor volume and eliminate micrometastases.2 Two randomized phase 3 clinical trials are under way to determine the efficacy of neoadjuvant chemotherapy plus surgery; however, until the results are published, the strategy is not recommended, especially at institutions with modern radiotherapy facilities (access to linear accelerators and intensity-modulated radiation therapy [IMRT] planning capabilities).
Alternative treatment strategies Two controversial alternative neoadjuvant chemotherapy strategies may be used, sometimes in an effort to conserve fertility in younger patients. One strategy involves chemotherapy followed by surgery or radiotherapy, relying on the chemotherapy agents to reduce tumor volume and eliminate micrometastases.2 Two randomized phase 3 clinical trials are under way to determine the efficacy of neoadjuvant chemotherapy plus surgery; however, until the results are published, the strategy is not recommended, especially at institutions with modern radiotherapy facilities (access to linear accelerators and intensity-modulated radiation therapy [IMRT] planning capabilities).
Neoadjuvant chemotherapy followed by radiotherapy, the other alternative strategy, may be more promising. A recent meta-analysis pooled data from 23 clinical trials. The results indicated that neoadjuvant cisplatin chemotherapy cycles delivered with dose intensities of 25 mg/m2/week or higher or for fewer than 15 days is associated with a survival advantage compared with lower dose-intense or longer chemotherapy schedules.2,6,9 Clinical trials, however, have not yet directly compared the outcomes of neoadjuvant chemotherapy and subsequent radiotherapy with the outcomes of standard concurrent chemoradiation.2 The potential benefits of postprimary treatment chemotherapy are also under investigation in current clinical trials; however, this strategy is not recommended unless posttreatment tumor persistence is noted.2
Overall, recent research has provided few prospects for substantive changes to the consensus recommendation of treating locally advanced cervical cancer with concurrent chemoradiation. The development of nanoparticulate radiosensitizers may somewhat improve chemoradiation outcomes, but this research is still in relatively early stages.10 “The progress I hope for is that HPV vaccination will drastically reduce the incidence of cervical cancer,” Verschraegen said. ONA
Bryant Furlow is a medical writer based in Albuquerque, New Mexico.
REFERENCES
1. SEER stat fact sheets: cervix uteri. Surveillance Epidemiology and End Results (SEER) Web site. http://seer.cancer.gov/statfacts/html/cervix.html. Accessed May 4, 2011.
2. Al-Mansour Z, Verschraegen C. Locally advanced cervical cancer: what is the standard of care? Curr Opin Oncol. 2010;22(5):503-512.
3. SEER cancer statistics review, 1975-2007. Surveillance Epidemiology and End Results Web site. http://seer.cancer.gov/csr/1975_2007/. Accessed May 4, 2011.
4. NCI issues clinical announcement on cervical cancer: chemotherapy plus radiation improves survival. http://www.nih.gov/news/pr/feb99/nci-22.htm. Accessed May 4, 2011.
5. Green J, Kirwan J, Tierney J, et al. Concomitant chemotherapy and radiation therapy for cancer of the uterine cervix. Cochrane Database Syst Rev. 2001;(4):CD002225.
6. Tzioras S, Pavlidis N, Paraskevaidis E, Ioannidis JP. Effects of different chemotherapy regimens on survival for advanced cervical cancer: systematic review and meta-analysis. Cancer Treat Rev. 2007;3(1)3:24-38.
7. Committee on Practice Bulletins-Gynecology. ACOG practice bulletin. Diagnosis and treatment of cervical carcinomas. Obstet Gynecol. 2002;99(5pt1):855-867.
8. Nugent EK, Case AS, Hoff JT, et al. Chemoradiation in locally advanced cervical carcinoma: an analysis of cisplatin dosing and other clinical prognostic factors. Gynecol Oncol. 2010;116(3):438-441.
9. Neoadjuvant Chemotherapy for Cervical Cancer Meta-Analysis Collaboration (NACCCMA Collaboration). Neoadjuvant chemotherapy for locally advanced cervix cancer. Cochrane Database Syst Rev. 2004;(2):CD001774.
10. Chithrani DB, Jelveh S, Jalali F, et al. Gold nanoparticles as radiation sensitizers in cancer therapy. Radiat Res. 2010;173(6):719-728.