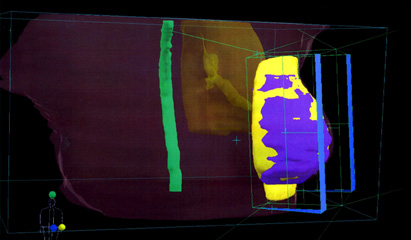
Adjuvant whole breast irradiation (WBI) after breast-conserving surgery has been shown to lower the risk of ipsilateral breast cancer recurrence by 70% at 5 years and to improve overall 15-year patient survival rates by 5%.1 Conventional WBI in the United States and Europe involves up to 28 treatments involving 1.8 to 2.0 Gy radiation doses per visit, usually with a subsequent radiation boost to the tumor bed.2 In the United Kingdom, however, hypofractionated WBI has been used for well over a decade to reduce the total number of patient treatment sessions and total treatment time, with comparable rates of tumor control and patient survival.3
NEW GUIDELINES DEVELOPED
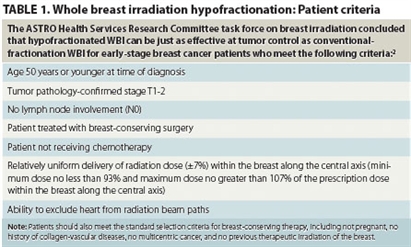
In August 2010, the American Society for Radiation Oncology (ASTRO) published WBI guidelines based on an ASTRO task force’s evidence-based systematic literature review of existing clinical studies. The task force concluded that—for early-stage breast cancer patients meeting specific criteria—WBI hypofractionation is as effective for tumor control and patient survival as conventional radiation therapy regimens.2 Patient criteria for radiation hypofractionation included, among others, age younger than 51 years; no lymph node involvement; and no concurrent chemotherapy. Table 1 gives the complete set of patient criteria used by the ASTRO task force.
These patient criteria represent populations for which sufficient clinical study data were available to support the use of hypofractionation. Patients who do not meet these criteria were poorly represented in the available empirical literature; and, the task force concluded, clinical evidence is therefore insufficient to determine the efficacy of hypofractionated radiation therapy regimens for those patients. The task force pointed out, however, that while additional clinical research is necessary to characterize the efficacy of hypofractionation for other patient populations, there does not exist compelling evidence that hypofractionation is actually ineffective or should be excluded from consideration for these patients. In the absence of compelling clinical evidence, task force members could not reach a consensus for or against the use of hypofractionated WBI for patients who do not meet the specified criteria.2
“Widespread adoption of HF-WBI for appropriately selected patients has the potential to enhance the convenience of treatment and lower the costs of WBI,” concluded Benjamin D. Smith, MD, lead author of the study and a radiation oncologist at the University of Texas M.D. Anderson Cancer Center in Houston. “For patients where the data to support HF-WBI are not as strong, HF-WBI can still be considered an option[;] but further research is needed.”
HYPOFRACTIONATION OF BREAST IRRADIATION
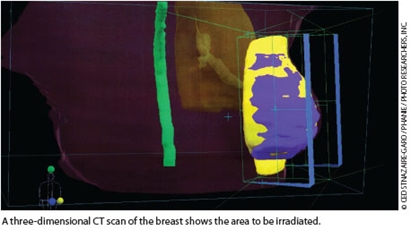 Administration of a patient’s total target radiation dose can be modified by altering fractionation of the dose—changing the number of irradiation sessions and the fraction of the dose delivered at each session.
Administration of a patient’s total target radiation dose can be modified by altering fractionation of the dose—changing the number of irradiation sessions and the fraction of the dose delivered at each session.
WBI fractionation schedules reflect two goals: the attempt to maximize the irradiation of tumor tissue over the shortest period of time possible (to reduce the risk of tumors developing reduced radiation sensitivity) while minimizing irradiation of healthy tissues around tumor margins and along beam pathways used to irradiate tumor tissue. Lower per-session fraction doses reduce the risk of late toxicity for healthy tissue while delivering a total radiation dose to tumor tissue sufficient to kill tumor cells.
Conventional fractionation entails drawbacks, such as inconvenience to the patient caused by repeated treatments over relatively long periods of time (6-7 weeks) and the consequently increased cost. Conventional WBI entails both economic costs (direct health care expenditures) and quality-of-life opportunity costs (lost patient time at work and home). Clinical data suggest that conventional fractionation may be unnecessarily conservative when radiation beam pathways do not include heart involvement.2
Hypofractionated radiation therapy is an alternative to conventional fractionation involving the administration of larger dose fractions less frequently, allowing patients to complete radiation therapy in 4 weeks or less.2 The ASTRO task force’s systematic review of randomized clinical trials in early-stage breast cancer patients published between 1990 and 2009—a literature resulting largely from direct comparisons of conventional and hypofractionated WBI conducted in the United Kingdom and Canada—concluded that hypofractionated schedules are as effective as conventional radiation regimens for patients who meet the criteria listed in Table 1.2 Efficacy outcomes analyzed included local tumor control, local-regional control, disease-free survival times, and overall survival rates, as well as toxicity measures.2 (In addition to 11 randomized clinical trials, the task force reviewed data from 35 nonrandomized clinical studies.2)
The task force’s guidelines recommend that patients not scheduled to receive a radiation boost should receive a hypofractionated dose schedule of 42.5 Gy in 16 fractions.2 Although data were insufficient to determine the indications for tumor bed radiation boost and resulting toxicities, the task force could not agree on the appropriateness of hypofractionated WBI when a boost is indicated. The task force agreed that hypofractionated WBI alone—without a boost dose—is inappropriate when a boost is indicated but could not agree on whether or not hypofractionation followed by a tumor bed boost is safe.2 A minority of task force members argued that conventional fractionation should be used when a boost is indicated.2
ASTRO’s hypofractionated WBI recommendations will expire in December 2015.2 A shift toward hypofractionated WBI for one of the most common cancers in the United States would have important implications both for patients and the US health care system. The sustainability of health care reform efforts will depend importantly on cost controls, a politically sensitive issue that has centered most controversially on concerns about the possible rationing of patient care. The identification of less costly treatment strategies with clinically equivalent patient outcomes, such as hypofractionated WBI, will benefit not only patients but also the US cancer care and health care systems in general.
TABLE 1. Whole breast irradiation hypofractionation: Patient criteria
REFERENCES
1. Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366:2087-2106.
2. Smith BD, Bentzen SM, Correa CR, et al. Fractionation for whole breast irradiation: An American Society for Radiation Oncology (ASTRO) evidence-based guideline. http://www.astro.org/Research/CommentForm/documents/FractionationDraft.pdf. Accessed August 20, 2010.
3. Whelan TJ, Levine M, Julian J, et al. The effects of radiation therapy on quality of life of women with breast carcinoma: results of a randomized trial. Ontario Clinical Oncology Group. Cancer. 2000;88:2260-2266.
Bryant Furlow is a medical writer living in Albuquerque, New Mexico.