A COSTLY PREVENTIVE
Six years after NIOSH’s 2004 recommendation that chemotherapy centers adopt CSTDs, several large US cancer centers contacted by Oncology Nurse Advisor had not yet done so. But Dr Spivey, having co-authored several studies of the PhaSeal and other CSTDs, is frequently consulted by hospitals looking into adopting one, and she says people contacting her lately have sounded more “serious” than in the past.
“We don’t currently use a closed system but we are in the process of reviewing them so that we can switch over,” University of New Mexico Cancer Center oncology pharmacist Stanley Cheshire, PharmD, said. “But it’s inevitable.” UNM is currently reviewing the sparse empirical literature of comparative studies to decide which of the available CSTD systems (described below) will best fit their needs.
“There are very few comparison articles,” noted Martin Martinez, PhD, who is heading up UNM’s CSTD search. Dr Martinez will be searching out and evaluating published and unpublished studies through March 2010, he said.
CSTDs’ steep costs remain a big barrier to widespread adoption, particularly in a cost-containment era—and will remain prohibitive for smaller centers into the near future, Dr Spivey believes. “CSTDs cost between $8 and $12 per dose,” she said. “PhaSeal’s the most expensive one. We find that our cost (with the PhaSeal system) is close to $12 per dose.” That comes to an average of $1.5 million a year at M. D. Anderson, noted Dr Spivey, and costs have not declined as additional manufacturers have joined the market with new products. “Smaller centers probably just aren’t going to be able to afford it,” she acknowledged.
There are not yet billing codes specific to CSTD equipment, and Medicaid and Medicare do not reimburse for the procedure. It primarily benefits health care workers rather than patients, which doesn’t fit neatly into Medicaid’s fee-for-patient-service approach to medical reimbursement. “We try to capture some of the charging in the IV patient bag charge,” Dr Spivey explained. “Manufacturers are working hard to get a (billing) code, and everything will explode at that point.”
AN EVOLVING EVIDENCE BASE
CSTDs are a young technology with competing designs and poor market penetration, and unfortunately, the meager published literature on these devices remains dominated by manufacturer-sponsored studies. No meta-analyses or systematic reviews of those studies were located in recent searches of several medical literature archives and databases.
Designs vary from physically closed systems with an expanding balloon to accommodate air pressure differentials (the PhaSeal approach), to compartmentalized isolators that employ sealed hoods with fixed gloves, to filter-based systems like the Tevadaptor that remove particles and vapors from air passing through the device. (Table 3 has a list of CSTD equipment and manufacturers.)
The largest comparative study to date was published in 2008 by Dr Spivey and colleagues at M. D. Anderson; Clarian Health of Indianapolis, Indiana; and the University of Utah in Salt Lake City (Table 4). Although the study itself was not sponsored by Carmel, Spivey told Oncology Nurse Advisor, both she and co-author James Jorgenson were at that time paid advisors to Carmel Pharma.
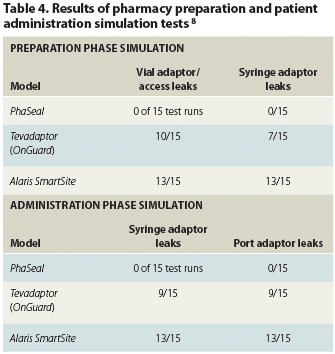 The team compared PhaSeal with four other CSTD models, testing whether or not titanium tetrachloride, a drug vapor simulant, escaped from connections between vial and syringe during preparation or from syringe and port during administration.8 When titanium tetrachloride contacts water particles in the air, it forms hydrochloric gas and titanium dioxide, which in turn form visible smoke. Photographs were taken of released smoke, indicating the probable escape of drug vapors. Four of the five systems tested leaked, with only Carmel’s PhaSeal evincing no escaped smoke.8
The team compared PhaSeal with four other CSTD models, testing whether or not titanium tetrachloride, a drug vapor simulant, escaped from connections between vial and syringe during preparation or from syringe and port during administration.8 When titanium tetrachloride contacts water particles in the air, it forms hydrochloric gas and titanium dioxide, which in turn form visible smoke. Photographs were taken of released smoke, indicating the probable escape of drug vapors. Four of the five systems tested leaked, with only Carmel’s PhaSeal evincing no escaped smoke.8
Dr Spivey and Howard Ritter at M. D. Anderson subsequently tested the dry vial/syringe connections and syringe/access port connections for three candidate CSTDs—PhaSeal, Tevadaptor (OnGuard), and Alaris SmartSite vented vial access device. They added a fluorescent indicator solution to empty 20 mL vials capped with a rubber stopper and vial cap. Photographing the vials under ultraviolet (UV) light to visualize leaks during syringe withdrawal and reinjection of indicator solution, simulating drug preparation in the pharmacy, and a 7 mL push using the syringe adaptor and IV port for each product to simulate administration to the patient, Dr Spivey and Ritter showed that, again, only PhaSeal’s dry connections prevented the escape of the fluorescent solution (Table 4).8
Leaking filters and membranes caused the observed leaks, Dr Spivey said. While all of the tested CSTDs likely reduce liquid or vapor escape during preparation and administration, the authors argue that only PhaSeal meets the strict definition of a truly closed (self-contained) drug transfer device that can prevent leaks.8 “PhaSeal’s more expensive than anything on the market, but we consider them the only true closed-system on the market,” Dr Spivey suggested.
But NIOSH contested that interpretation, stating in a letter to Carmel Pharma that the NIOSH’s definition of CSTDs was intended only to require preservation of the sterility of the product and preventing escape of hazardous drugs into the environment.10 Moreover, it seems studies employing different simulants may yield different end results in comparative studies. A study sponsored by the manufacturer of Tevadaptor found that in tests using Technetium-99 simulant, the Tevadaptor meets the NIOSH definition of CSTD.11
No CSTD is a magic bullet, Spivey was quick to add. CSTDs should always be used in the context of BSCs and vigilant attention to OSHA and NIOSH guidelines, she emphasized.