Stereotactic radiosurgery (SRS) and stereotactic body radiation therapy (SBRT) are increasingly widely used external irradiation modalities for cancer treatment. They represent rapidly developing treatments that precisely and noninvasively target narrow radiation beams from different angles to maximize radiation dose to tumor tissue while minimizing the dose to healthy tissues in the beam paths.
Both SRS and SBRT are devised to deliver higher radiation doses to smaller radiation fields around solid tumors, allowing delivery of complete therapeutic doses in fewer sessions than is possible with other, less precisely targeted (and therefore more fractionated) radiotherapy techniques. Fractionation involves breaking up the total therapeutic dose of radiation over a series of treatment sessions to minimize non-target tissue irradiation and to allow healthy, incidentally-irradiated tissue time to recover between dose fractions. Delivering the total therapeutic radiation dose over a shorter period of time helps reduce the risk of tumor cells developing resistance to radiation damage, although tumor radioresistance remains a leading cause of radiosurgical failure.1
Three SRS and SBRT technologies are in use: particle beam proton SRS; cobalt 60 (Gamma Knife) SRS and linear accelerator based (linac) SRS.2 Gamma Knife and linac units are widely available across the United States.2
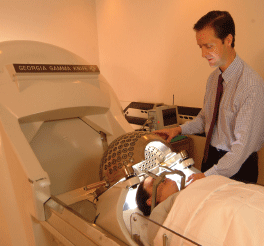
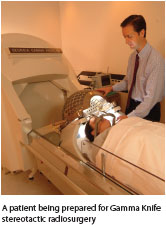 SRS delivers one or two high-dose radiation exposures to small tumors(3.0 cm or smaller) using patient positioning techniques and increasingly sophisticated image guidance systems to ensure precise radiation targeting of tumor tissue. Because of the critically important role of image guidance and the importance of avoiding nontarget irradiation, SRS is used only with tumors that have well-defined margins, such as primary or metastatic spinal tumors or brain tumors—particularly astrocytomas and noninfiltrating gliomas, the latter being the most common primary brain tumors.2-6 SRS is widely used for the treatment of brain and spinal tumors that would be too dangerous to treat with traditional surgical resection. SRS is also used to treat benign brain tumors that are potentially life-threatening, such as benign meningiomas, pituitary tumors, pineal tumors, and acoustic neuromas, as well as non-cancer-related functional disorders like Parkinson’s tremor and multiple sclerosis.2,7
SRS delivers one or two high-dose radiation exposures to small tumors(3.0 cm or smaller) using patient positioning techniques and increasingly sophisticated image guidance systems to ensure precise radiation targeting of tumor tissue. Because of the critically important role of image guidance and the importance of avoiding nontarget irradiation, SRS is used only with tumors that have well-defined margins, such as primary or metastatic spinal tumors or brain tumors—particularly astrocytomas and noninfiltrating gliomas, the latter being the most common primary brain tumors.2-6 SRS is widely used for the treatment of brain and spinal tumors that would be too dangerous to treat with traditional surgical resection. SRS is also used to treat benign brain tumors that are potentially life-threatening, such as benign meningiomas, pituitary tumors, pineal tumors, and acoustic neuromas, as well as non-cancer-related functional disorders like Parkinson’s tremor and multiple sclerosis.2,7
When SRS involves more than one dose, it is sometimes referred to as stereotactic radiotherapy (SRT). SRT is distinguished from SRS by multiple treatment doses and is typically employed against larger tumors, whereas SRS is ideally employed against small, well-defined tumors.
SRS can be used on patients who have previously undergone whole brain radiation. Because SRS minimizes the irradiation of nontarget tissue, the cognitive impacts of treatment have long been argued to be less pronounced than those reported for whole-brain irradiation, leading some authors to argue that SRS should be preferentially used as a first-line treatment for brain tumors.1 However, a recent systematic review of 16 studies published between 2002 and 2007 raises doubts about this logical assumption; the authors found that SRS plus whole-brain radiotherapy provided better local control of metastatic brain tumors—and, counterintuitively, neurologic function—than either treatment modality alone.4
SBRT, also commonly known by the brand name CyberKnife, refers to radiotherapy technique similar to SRS but targeting tissues outside the brain and spine. SBRT is currently used primarily to treat small, isolated tumors in the liver and lungs, and more recently, thanks to image guidance motion compensation, the urinary bladder.3,8 Because lung, bladder, and other body tissues tend to move with normal patient respiration and other bodily functions, computer image tracking is used to compensate or correct for target motion during irradiation. Nevertheless, targeting cannot yet be as precise as is the case with SRS, and total radiation dose is therefore fractionated so that SBRT is delivered in a series of at least two doses.3
Both SRS and SBRT rely on three-dimensional (3-D) targeting and pretreatment imaging with diagnostic imaging modalities, typically CT and MRI, to identify target tissue margins and anatomic landmarks. Computer-based stereotactic planning systems build a 3-D atlas of the tumor and surrounding, healthy organ tissue.
PATIENT POSITIONING AND LANDMARKS
Patient positioning and fixation remain important, but image-guided strategies are increasingly sophisticated. Traditionally, patient-positioning devices such as head clamps are used to fix the target organ (eg, the brain) into position. SRT and SBRT involve using masks or frames rather than the surgically fixed frame attached by pins to the skull that is used for SRS. Frameless imaging-guided thermoplastic masks are used instead of frames more often.9
Anatomic landmarks are still widely used with SRS, based on diagnostic imaging-derived atlases. For SBRT, gold markers surgically implanted prior to therapy are used as landmarks.
SIDE EFFECTS
Radiation edema sometimes occurs as irradiated tumors become unable to regulate fluid homeostasis and is treated with corticosteroids.2 Radiation necrosis and tumor radioresistance are the leading causes of SRS failure; however, reliable predictions of which tumors will develop radioresistance are not yet possible.1 Brainstem SRS can cause radiation injury to healthy tissue because of the irregular shape of brainstem tumors; a recent study reported radiation injury in 4% of brainstem SRS patients.10 A case of optic neuritis was recently reported as a rare adverse effect of SRS for pituitary adenoma, manifesting as elevated serum prolactin and headaches.11
COMING ADVANCES
Targeting accuracy has improved over time and will continue to do so in the years to come, making SBRT an attractive cancer treatment option for an expanding number of organ systems. The intercorrelation of multiple imaging datasets allows for more precisely planned SRS and SBRT; and 4-D planning systems that will better allow for target changesover short periods, such as organ motion, and inter-treatment tumor volume reduction in SRT and SBRT treatments involving more than one radiation dose are in development.12 Integrated, high-speed imaging feedback and motion-compensation systems promise improved targeting. Radioprotectants (such as amifostine) and radiosensitizers—which make tumor tissue more vulnerable to radiation and surrounding healthy tissue less radiosensitive, thereby minimizing radiation injury to nontarget tissue—are also being developed.12 ONA
Bryant Furlow is a medical writer living in Albuquerque, New Mexico.
REFERENCES
1. Jagannathan J, Bourne TD, Schlesinger D, et al. Clinical and pathological characteristics of brain metastasis resected after failed radiosurgery. Neurosurgery. 2010;66(1):208-217.
2. International RadioSurgery Association (IRSA). Stereotactic radiosurgery overview. IRSA Web site. www.irsa.org/radiosurgery.html. Accessed August 2, 2010.
3. National Cancer Institute. Stereotactic radiosurgery. www.cancer.gov/cancertopics/factsheet/Therapy/radiation. Accessed August 2, 2010.
4. Müller-Riemenschneider F, Bockelbrink A, Ernst I, et al. Stereotactic radiosurgery for the treatment of brain metastases. Radiother Oncol. 2009;91(1):67-74.
5. Pollock BE. Stereotactic radiosurgery of benign intracranial tumors. J Neurooncol. 2009;92(3):337-343.
6. Fogh SE, Andrews DW, Glass J, et al. Hypofractionated stereotactic radiation therapy: an effective therapy for recurrent high-grade gliomas. J Clin Oncol. 2010;28(18):3048-3053.
7. Castinetti F, Régis J, Dufour H, Brue T; Medscape. Role of stereotactic radiosurgery in the management of pituitary adenomas. Nat Rev Endocrinol. 2010;6(4):214-223.
8. Thariat J, Trimaud R, Angellier G, et al. Innovative image-guided CyberKnife stereotactic radiotherapy for bladder cancer. Br J Radiol. 2010;83(990):E118-E121.
9. Ramakrishna N, Rosca F, Friesen S, et al. A clinical comparison of patient setup and intra-fraction motion using frame-based radiosurgery versus a frameless image-guided radiosurgery system for intracranial lesions. Radiother Oncol. 2010;95(1):109-115.
10. Shipani S, Jain R, Shah K, et al. Clinical, dosi-metric, and radiographic correlation of radiation injury involving the brainstem and the medial temporal lobes following stereotactic radiotherapy for neoplasms of the central skull base. J Neurooncol. 2010;98(2):177-184.
11. Daniels TB, Pollock BE, Miller RC, et al. Radiation-induced optic neuritis after pituitary adenoma radiosurgery in a patient with multiple sclerosis: case report. J Neurooncol. 2009;93(2):263-267.
12. Ma L, Sneed PK. The future of radiosurgery and radiotherapy. In: Lozano AM, Gildenberg PL, Tasker RR, eds. Textbook of Stereotactic and Functional Neurosurgery. Berlin, Germany: Springer; 2009:3143-3154.