Eating habits can change dramatically for cancer patients. Loss of appetite; the effects of treatment on digestion; changing nutritional demands; the timing of meals relative to administration of medications; and dietary supplements taken by patients as folk remedies or to treat the symptoms of cancer and side effects of chemotherapy or radiation treatments all play important roles in patients’ dietary shifts. Cancer can trigger cachexia and malnutrition, compoundedor hastened by nausea, vomiting, anorexia, or constipation.
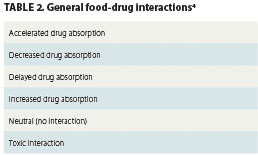
An often-neglected facet of the diet-cancer treatment equation is food-treatment interaction, or the impact dietary practices can have on the efficacy of chemotherapy or radiation therapy. Drugs can affect nutritional states, but foods can also significantly modulate the bioavailability and absorption time profiles of chemotherapeutic drugs, potentially in ways that could reduce the efficacy of the medications (Table 1). Interactions may be driven by the chemistry of the food, beverage, or dietary supplement on a drug’s bioavailability; increased or, more frequently, decreased metabolic rates after meals; reduced digestive tract pH soon after meals; and the effects of food fats on drug absorption in the digestive tract (Table 2). The effects of patients’ dietary practices on drug absorption and efficacy have been proposed as one explanation for the wide variation in toxicity and treatment outcomes experienced by cancer patients.1
DRUG INTERACTIONS WITH FOOD AND MEALS
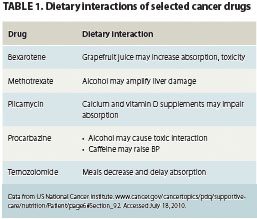 Food and drug chemistry, timing and composition of meals, and meal size can all affect drug bioavailability. Recent meals can interfere with the bioavailability of oral chemotherapy drugs, such as lapatinib (used to treat metastatic breast cancer or other solid tumors) or nilotinib (used to treat chronic myeloid leukemia [CML]).
Food and drug chemistry, timing and composition of meals, and meal size can all affect drug bioavailability. Recent meals can interfere with the bioavailability of oral chemotherapy drugs, such as lapatinib (used to treat metastatic breast cancer or other solid tumors) or nilotinib (used to treat chronic myeloid leukemia [CML]).
The fat content of food eaten may magnify the impact of a meal on absorption times and absorbed doses of these drugs.2,3 High-fat food may slow the absorption time of nilotinib in patients with CML by as much as 50%. Nilotinib should be taken on a minimum 2-hour fast only, and the patient should fast for 1 hour after taking the drug.3 Taking oral chemotherapy drugs with soft drinks or fruit juices, particularly acidic grapefruit or orange juice, can alter stomach and intestinal pH and transporter protein activity in the digestive tract, potentially increasing or decreasing the drug’s absorption rate.4 Many chemotherapeutic drugs have narrow windows between absorption of therapeutic levels and minimum toxic doses and may therefore be particularly affected by some dietary practices.1
Much of the recent literature on the interactions of diet and dietary supplement use with cancer treatment is anecdotal. Studies are typically based on findings among small numbers of patients, and proposed food-drug interactions are frequently based on preclinical laboratory findings rather than clinical studies.5 Conclusions about clinical implications are therefore rarely without controversy.
 A recent search of the medical literature identified no meta-analyses on specific food-cancer drug interactions, and no systematic framework exists for predicting food-drug interactions. The best nurses can do for their patients is to identify associations between specific drugs and foods or supplements, or between drugs and the timing of meals. Communicating food-drug interactions to patients may be even more critical for the elderly than for younger patients because aging processes also alter drug absorption profiles, increasing the risk of insufficient dosing or overdosing.4
A recent search of the medical literature identified no meta-analyses on specific food-cancer drug interactions, and no systematic framework exists for predicting food-drug interactions. The best nurses can do for their patients is to identify associations between specific drugs and foods or supplements, or between drugs and the timing of meals. Communicating food-drug interactions to patients may be even more critical for the elderly than for younger patients because aging processes also alter drug absorption profiles, increasing the risk of insufficient dosing or overdosing.4
Many claims in the popular media regarding anticancer diets and dietary or diet-supplement cancer cures are extremely speculative and poorly supported by empirical evidence. Curative claims for diets should not be confused with diets intended to improve cancer patients’ nutritional status and comfort during treatment; the latter can make a significant contribution toward improved quality of life for patients (Table 3).
POPULAR DIETARY SUPPLEMENTS
Unproven diets and dietary supplements, such as garlic, mistletoe, Essiac, Lingzhi, St. John’s wort, and astragalus, are popular among cancer patients.6 For example, a recent survey in the United Kingdom found that 22% of patients with newly-diagnosed cancer used herbal supplements.7 These supplements are frequently self-administered by patients in the hope of controlling tumor growth or to reduce the toxic side effects of prescribed chemotherapies.6
 Although some dietary supplements, such as cod liver oil or fish oil, appear to be associated with reduced cancer risk and improved quality of life (eg, improved depression), available empiric evidence of the benefits of most supplements remains very weak. Sadly, clinical research suggests some supplements can interfere with chemotherapy (Table 4). St. John’s wort reduces the concentration and bioavailability of the chemotherapeutic metabolite of irinotecan (SN-38) by 42% and imatinib by 32%.6
Although some dietary supplements, such as cod liver oil or fish oil, appear to be associated with reduced cancer risk and improved quality of life (eg, improved depression), available empiric evidence of the benefits of most supplements remains very weak. Sadly, clinical research suggests some supplements can interfere with chemotherapy (Table 4). St. John’s wort reduces the concentration and bioavailability of the chemotherapeutic metabolite of irinotecan (SN-38) by 42% and imatinib by 32%.6
Antioxidant supplements remain controversial; widely used for their reported anticancer properties, they may actually be counterproductive for cancer patients, possibly protecting tumor cells from molecular damage from radiation therapy and chemotherapy.8 Vitamin C (ascorbic acid) is an antioxidant that has been tied to reduced clinical activity of the antimyeloma drug bortezomib in a human xenograft mouse model of myeloma, prompting a recent recommendation that vitamin C supplements be avoided during bortezomib therapy.9
