Malignant melanoma was newly diagnosed in 68,720 patients in 2009, caused 8,650 deaths that year, and up to 18% of patients had regional or distant metastasis at the time of diagnosis.1 Melanoma has long been deemed a highly radioresistant malignancy, discouraging clinical studies of radiotherapy for patients with this deadly disease. Early preclinical studies found that malignant melanoma cells better survive irradiation than other cancer cells. However, more recent preclinical studies have found a range of radiosensitivities in different melanoma cell lines, leading some researchers to argue that a historical fluke—the use of particularly radioresistant cell lines in early studies—caused undue skepticism about the promise of radiotherapy for melanoma.2,3 Furthermore, critics pointed out early clinical studies that seemed to confirm melanoma’s belligerent radioresistance employed outdated radiotherapy procedures and research designs, leaving their results and applicability to contemporary radiotherapy unclear.2
Recent studies suggest a clinically meaningful role for adjuvant external-beam radiotherapy in the treatment of lymph-node-involving melanoma and plaque brachytherapy for melanoma of the eye.1 In light of the high rate of relapse after surgical lymphadenectomy for advanced melanoma, the role of adjuvant radiotherapy has recently received particular attention.1,4,5
ADJUVANT RADIOTHERAPY REDUCES NODE-FIELD RELAPSE
A randomized, controlled international clinical trial found that adjuvant postsurgical nodal basin radiotherapy (48 Gy in 20 fractions; n=109 patients) was associated with a significant reduction in lymph-node field relapse compared to surgery alone (n=108 patients), after a median follow-up time of 40 months.4 Relapse occurred in 20 patients who underwent adjuvant radiotherapy versus 34 patients who had only surgery (hazard ratio [HR], 0.56; 95% confidence interval [CI], 0.32-0.98; P=.04).4 Toxic effects were manageable, with grade 3 and 4 adverse events including radiation dermatitis (19 in the radiotherapy group), wound infection (three in the radiotherapy group vs seven in the surgery-only group), and seroma fluid accumulations (nine in the radiotherapy group, 11 in the surgery-only group).4
Unfortunately, adjuvant radiotherapy exhibited no improvement over surgery alone for relapse-free survival rates, and an unexplained and statistically nonsignificant trend toward decreased overall survival rates (59 deaths vs 47 deaths, respectively; HR, 1.37; 95% CI, 0.94-2.01; P=.12).4 Nevertheless, the authors conclude adjuvant radiotherapy “should be discussed with patients at high risk of relapse after lymphadenectomy.”4 Risk stratification using the number and size of melanoma-affected lymph nodes and the presence of extracapsular tumor tissue might allow for identification of patients most likely to benefit from adjuvant radiotherapy.4,5
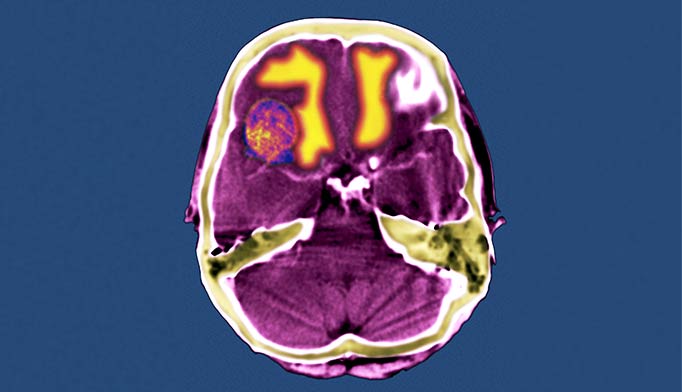
RADIOTHERAPY IN BRAIN METASTASIS
The potential role of radiotherapy in prolonging the lives of patients with even the most challenging forms of melanoma, brain metastases, is also receiving new attention. Brain metastases is found in up to 40% of patients with melanoma in clinical study populations and up to 72% of patients with metastatic melanoma at autopsy.6 Median overall survival time is 3.8 months.7 Utilization of adjuvant whole-brain radiotherapy (WBRT) varies dramatically; some cancer centers encourage WBRT, and some do not even offer this option to patients with melanoma.6 Recruiting adequate numbers of patients with metastatic melanoma for clinical trials has proven challenging, particularly for trials involving WBRT, limiting the evidence base for optimal treatment planning.6,8
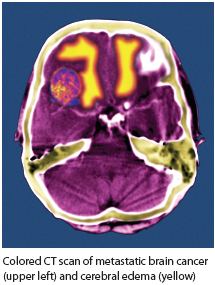 As with other early studies of nodal melanoma radiotherapy, most studies of WBRT for patients with brain metastases of melanoma have been retrospective, and involved inconsistent treatment plans or different patient populations, complicating meaningful comparisons of outcomes.2,9 No prospective clinical trials of adjuvant WBRT (following stereotactic radiosurgery) have been published for populations of patients with brain metastases of melanoma.9 Authors of WBRT studies that have included small numbers of patients with brain metastases of melanoma have sometimes drawn conclusions supporting the skeptical consensus about radiation’s promise for this disease, but critics have pointed out that frequently too few melanoma patients were included in those studies to allow for sufficient statistical power to infer reliable conclusions about outcomes. One study included only seven patients with brain metastases of melanoma, for example; another, only 16 such patients received WBRT.9 Despite a similarly small sample size of 34 patients, results from one early retrospective WBRT study did suggest a statistically significant benefit for patients who received both surgical metastectomy and adjuvant WBRT compared with surgery alone.9,10 That study excluded patients with existing extracranial melanoma or more than one metastatic melanoma tumor in the brain.10
As with other early studies of nodal melanoma radiotherapy, most studies of WBRT for patients with brain metastases of melanoma have been retrospective, and involved inconsistent treatment plans or different patient populations, complicating meaningful comparisons of outcomes.2,9 No prospective clinical trials of adjuvant WBRT (following stereotactic radiosurgery) have been published for populations of patients with brain metastases of melanoma.9 Authors of WBRT studies that have included small numbers of patients with brain metastases of melanoma have sometimes drawn conclusions supporting the skeptical consensus about radiation’s promise for this disease, but critics have pointed out that frequently too few melanoma patients were included in those studies to allow for sufficient statistical power to infer reliable conclusions about outcomes. One study included only seven patients with brain metastases of melanoma, for example; another, only 16 such patients received WBRT.9 Despite a similarly small sample size of 34 patients, results from one early retrospective WBRT study did suggest a statistically significant benefit for patients who received both surgical metastectomy and adjuvant WBRT compared with surgery alone.9,10 That study excluded patients with existing extracranial melanoma or more than one metastatic melanoma tumor in the brain.10
Recurrence of brain metastases occurred in five of 22 patients receiving surgery plus WBRT, compared with nine of 12 patients receiving metastasectomy alone.10 In that study, median overall survival in patients who received adjuvant WBRT was 3 times longer than in those who received only surgery (18 months vs 6 months, respectively; P=.002).9,10 Whether these promising results apply generally to patients with brain metastases of melanoma is not yet clear because few patients with brain metastases have a lone brain tumor and no active extracranial melanoma; however, the results do suggest that adjuvant WBRT with surgery might allow treatment plans to focus on extracranial tumor control and patient participation in clinical trials.9
A multicenter randomized phase III clinical trial of WBRT led by the Australia and New Zealand Melanoma Trials Group is recruiting patients in English-speaking countries, and its findings may answer longstanding questions about the optimal planning of radiotherapy for these patients. A full study protocol can be obtained from the principal investigator, Gerald Fogarty ([email protected]).
FUTURE ROLES IN MANAGING MELANOMA
The optimal roles and modalities for radiotherapy in the arsenal of existing and emerging tools for the management of high-risk melanoma have not yet been determined. The high energy transfer at the distal end of proton radiotherapy’s Bragg peak has also shown some preliminary preclinical promise for killing human melanoma cells.11 Targeted chemotherapies for melanoma are promising, and early preclinical findings hint at possible synergistic effects with radiotherapy. For example, the BRAF inhibitor vemurafenib (Zelboraf) appears to radiosensitize BRAF mutation-positive melanoma cells.3,5 Radiotherapy plus the human monoclonal antibody ipilimumab (Yervoy) combination therapy is also under investigation.5 ONA
Bryant Furlow is a medical journalist based in Albuquerque, New Mexico.
REFERENCES
1. Khan N, Khan MK, Almasan A, et al. The evolving role of radiation therapy in the management of malignant melanoma. Int J Rad Oncol Biol Phys. 2011;80(3):645-654.
2. Cranmer LD. Melanoma’s radioresistant reputation challenged. Oncol (Williston Park). 2010;24(7):656.
3. Sambade MJ, Peters EC, Thomas NE, et al. Melanoma cells show a heterogeneous range of sensitivity to ionizing radiation and are radiosensitized by inhibition of B-RAF with PLX-4032. Radiother Oncol. 2011;98(3):394-399.
4. Burmeister BH, Henderson MA, Ainslie J, et al. Adjuvant radiotherapy versus observation for patients at risk of lymph node field relapse after therapeutic lymphadenectomy for melanoma: a randomised trial. Lancet Oncol. 2012;13(6):589-597.
5. Macklis R. Finally, a substantial role for radiotherapy in melanoma. Lancet Oncol. 2012;13(6):561-562.
6. Fogarty G, Morton RL, Vardy J, et al. Whole brain radiotherapy after local treatment of brain metastases in melanoma patients—a randomised phase III trial. BMC Cancer. 2011;11:142. http://www.biomedcentral.com/1471-2407/11/142. Accessed October 9, 2012.
7. Sampson JH, Carter JH Jr, Friedman AH, Seigler HF. Demographics, prognosis and therapy in 702 patients with brain metastases from malignant melanoma. J Neurosurg. 1998;88(1):11-20.
8. Chang EL, Hwu P. Whole-brain radiation therapy in melanoma: an open question [authors’ reply]. Lancet Oncol. 2010;11(1):13-14.
9. Cranmer LD, Jeter JM, Morgan SS, et al. Whole-brain radiation therapy in melanoma: an open question. Lancet Oncol. 2010;11(1):13.
10. Skibber JM, Soong SJ, Austin L, et al. Cranial irradiation after surgical excision of brain metastases in melanoma patients. Ann Surg Oncol. 1996;3(2):118-123.
11. Petrović I, Ristić-Fira A, Todorović D, et al. Response of a radioresistant human melanoma cell line along the proton spread-out Bragg peak. Int J Radiat Biol. 2010;86(9):742-751.