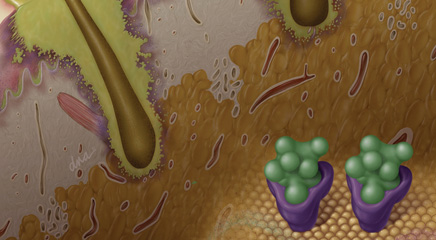
Radiotherapy is increasingly administered in combination with targeted biologic anticancer agents such as epidermal growth factor receptor (EGFR) inhibitors. Such combination therapies represent important advances in individually tailored treatment strategies that can improve patient survival times.1,2 But with these advances have also emerged unanticipated and increasingly common dermatologic toxicities that can include disfiguring and debilitating toxicities of the face, neck, and oral mucosa.1,3,4 Oncology nurses play a central role in monitoring and educating patients, and detecting and managing these toxicities; they should be familiar with new treatment regimens’ toxicities and management.
Dermatologic toxicities associated with radiotherapy-concurrent EGFR blockade can cause discomfort and pain, complicate chewing and swallowing, and increase risk of infection, impacting patients’ nutritional and functional status and potentially increasing the likelihood of treatment interruption or suboptimal dose delivery.1 Because of the potential impact on patient quality of life, dose intensity, and treatment outcome, detection and management of dermatologic toxicities associated with combination radiotherapy are receiving increasing attention.1 One example is radiotherapy-concurrent EGFR inhibition therapy with cetuximab (Erbitux), a particularly promising treatment strategy that significantly improves overall 5-year survival rates for patients with locally advanced squamous cell carcinoma of the head and neck (HNSCC) compared with radiotherapy alone (5-year survival rates of 45.6% vs 36.4%, respectively).5 A chimeric mouse/human monoclonal antibody administered intravenously for metastatic colorectal and locally advanced or metastatic HNSCC, cetuximab—and other EGFR inhibitors including erlotinib [Tarceva], panitumumab [Vectibix], and gefitinib [Iressa]—is associated with a high incidence of low-grade dermatologic toxicities even when administered as a monotherapy. Cutaneous reactions affect more than 50% of patients undergoing anti-EGFR therapy, but importantly, these rarely reach grade 3/4 in monotherapy settings.3
CHANGE IN STUDY FINDINGS
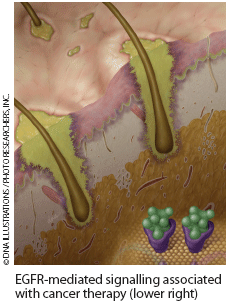 Early clinical trials failed to detect a significantly elevated risk of high-grade dermatologic toxicity from radiotherapy-concurrent cetuximab for HNSCC.2 But case reports and studies have confirmed that this combination radiotherapy is associated with high rates of severe, acneiform-rash-exacerbated radiation dermatitis and skin necrosis or ulceration with spontaneous bleeding within radiation portals, severe oral inflammation, and acneiform skin rash outside radiation portals.1,3,6-9
Early clinical trials failed to detect a significantly elevated risk of high-grade dermatologic toxicity from radiotherapy-concurrent cetuximab for HNSCC.2 But case reports and studies have confirmed that this combination radiotherapy is associated with high rates of severe, acneiform-rash-exacerbated radiation dermatitis and skin necrosis or ulceration with spontaneous bleeding within radiation portals, severe oral inflammation, and acneiform skin rash outside radiation portals.1,3,6-9
One recent study of 99 HNSCC patients found that grade 3/4 dermatitis developed in 34% of patients receiving concurrent cetuximab and IMRT radiotherapy compared with 3% of patients receiving cisplatin concomitant with radiotherapy, a 10-fold higher risk (P <0.01).10 A 2009 survey of 28 hospitals in 11 European countries found that 49% of HNSCC patients treated with radiotherapy-concurrent cetuximab developed grade 3/4 radiation dermatitis.8 Radiotherapy-concurrent cetuximab therapy is also associated with high rates of severe cheilitis (inflammation of the lip; 26%) and anterior stomatitis (38%), despite a low radiation dose to the lips and anterior oral cavity (median maximum dose to lips, 9.3 Gy; anterior oral cavity, 20 Gy).7 High-grade cheilitis is more often seen among HNSCC patients who developed severe acneiform rash.7
EGFR inhibition may cause amplified inflammatory response and epithelial cell death; however, the exact mechanisms responsible for higher dermatologic toxicity risks among patients receiving radiotherapy-concurrent cetuximab remain unclear, particularly for rashes occurring outside radiation portals.
EMERGING GUIDELINES
Anti-EGFR-associated severe radiation dermatitis and associated dermatologic toxicities are likely to become more frequent as combination therapies are more widely administered to patients with HNSCC and other cancers. Large, randomized, clinical-trial-based management guidelines specific to toxicities associated with radiotherapy-concurrent EGFR blockade are not yet available, but expert consensus guidelines were recently published.9,11,12
Once established, evidence-based guidelines for optimal management of toxicities associated with radiotherapy-concurrent EGFR blockade may be different and distinct from best practices for similar toxicities associated with other treatments. For example, prophylactic topical corticosteroid therapy is commonly recommended (or described as not contraindicated) for patients receiving radiotherapy-concurrent cetuximab for prevention or management of radiation dermatitis and other dermatologic toxicities.9,11,13 However, because corticosteroids can exacerbate acneiform rash progression among patients receiving anti-EGFR therapy in nonradiotherapy settings, some authors have cautioned that corticosteroids may cause more severe skin damage in patients receiving radiotherapy-concurrent anti-EGFR therapy than occurs in other patient populations.14
MANAGING RADIATION DERMATITIS
As with all patients receiving radiotherapy, patients should be educated to keep irradiated skin clean and dry, even when ulcerated, using gentle washing and drying technique.9 When moderate to severe radiation dermatitis does occur, secondary bacterial infection, usually involving Staphylococcus aureus, can be common.9 If infection is suspected, blood cultures should be ordered to exclude the possibility of sepsis if the patient has fever or a low neutrophil count.9