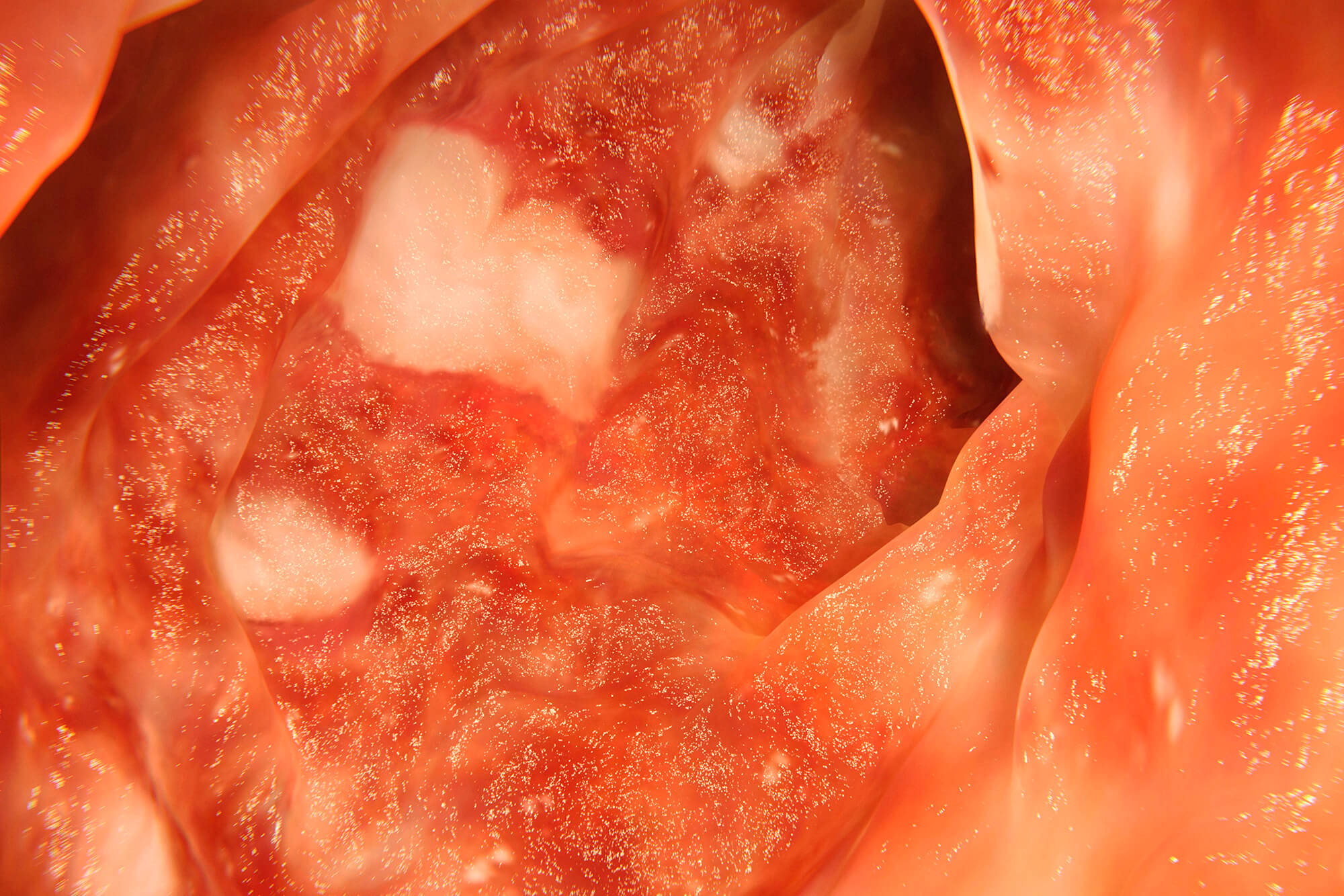
Colitis associated with rituximab therapy in patients with cancer is usually mild and treatable with supportive care but occasionally can be severe, according to results from an analysis published in the American Journal of Clinical Oncology.
In this study, researchers retrospectively assessed gastrointestinal toxicity associated with rituximab therapy in 1660 patients with cancer who had undergone a colonoscopy between 2000 and 2018. This study excluded patients with competing etiologies for colitis.
A total of 4% (70/1660) patients developed rituximab-associated colitis. The median time from initiation of rituximab therapy to onset of colitis was 181 days.
Clinical GI symptoms occurred in 53 patients, with 39 cases of diarrhea, 19 cases of abdominal pain, 11 cases of blood per rectum, and 5 cases of concurrent fever. Symptoms persisted a median of 21 days.
Of the 70 patients with rituximab-associated colitis, 71% (50/70) received therapy, with antimotility agents used in 42 patients, supportive care in 42 patients, antimicrobial agents in 21 patients, and immunosuppressive treatment in 12 patients.
Mucosal ulceration on endoscopy occurred in 9 patients. Characteristics of active4 inflammation per histological analysis occurred in 52 patients. Hospital admission was required for 39 patients, with 2 patients admitted to the intensive care unit.
Patients with abnormal endoscopic results experienced more frequent hospitalization (P =.024) and increased therapy for rituximab-associated colitis (P =.001).
Though these results indicate rituximab-associated colitis is typically mild and require only supportive care, occasionally it can be severe and result in perforation of the colon and admission to the intensive care unit.
The researchers suggested that rituximab-associated colitis “should be considered among the differential diagnoses in patients who received rituximab therapy and present with GI [gastrointestinal] symptoms for up to 2 years after patients receive the last dose of rituximab.”
Reference
Mallepally N, Abu-Sbeih H, Ahmed O, et al. Clinical features of rituximab-associated gastrointestinal toxicities. Am J Clin Oncol. 2019;42(6):539-545.