PDF of CE 0710 Revised
HOW TO TAKE THE POST-TEST: To obtain CE credit, please click here after reading the article to take the post-test on myCME.com.
An estimated 1,399,790 new cancer diagnoses were made in the United States in 2006. Most of these cancers required treatment with radiation and/or chemotherapy, interventions that help 65% of persons with cancer to survive at least 5 years after their initial diagnosis.1 The development of novel targeted anticancer agents, such as the tyrosine kinase inhibitors (TKIs)—whether these are used as monotherapy or in combination regimens with chemotherapy—has contributed to improved quality of life and longer survival for cancer patients.1,2 Prolonged survival has in turn underscored the significance of managing emotional, social, and medical problems as integral components of continued cancer care.1
The use of long-term cancer treatments has caused an unexpected constellation of side effects to emerge—most notably the cutaneous toxicities.1 These are becoming more prevalent with increased use of targeted therapies in addition to traditional chemotherapeutic agents.3 Of the cutaneous toxicities experienced by patients, hand-foot syndromes (HFS)—also known as palmar-plantar erythrodysesthesia, palmar-plantar erythema, acral erythema, and Burgdorf’s reaction—are becoming among the most common.1-3
Certainly not a new phenomenon, HFS has been reported in 6% to 42% of patients treated with traditional systemic chemotherapeutic agents such as 5-fluorouracil (5-FU, Adrucil,) and its analogs, doxorubicin (Adriamycin, Doxil, Rubex), cytarabine (Cytosar-U, Depo-Cyt), cyclophosphamide (Cytoxan, Neosar), vinorelbine (Navelbine), and docetaxel (Taxotere), as well as in those treated with targeted therapies such as sorafenib (Nexavar) and sunitinib (Sutent).4-10 The incidence of HFS observed in clinical trials with agents such as capecitabine (Xeloda) is around 50%, with 17% of patients reporting a severe form (grade 3).6
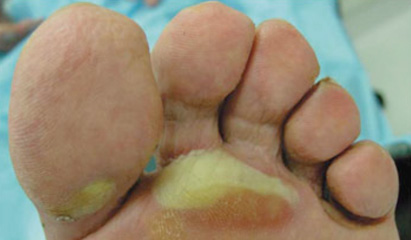
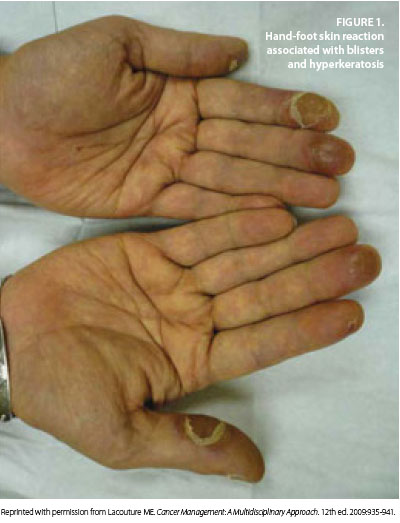 Adding to this surge of HFS incidence has been the development of novel oral targeted therapies with efficacy against advanced renal cell carcinoma, hepatocellular carcinoma, gastrointestinal stromal tumor, and other tumor types.2,10 These therapies include the TKIs sorafenib and sunitinib, which are notable for causing hand-foot skin reactions (HFSR) in 20% to 40% of treated patients (Figure 1 and Figure 2). HFSR is a distinct variant of the more widely known HFS, which occurs with more traditional chemotherapy agents such as capecitabine.1-4, 10
Adding to this surge of HFS incidence has been the development of novel oral targeted therapies with efficacy against advanced renal cell carcinoma, hepatocellular carcinoma, gastrointestinal stromal tumor, and other tumor types.2,10 These therapies include the TKIs sorafenib and sunitinib, which are notable for causing hand-foot skin reactions (HFSR) in 20% to 40% of treated patients (Figure 1 and Figure 2). HFSR is a distinct variant of the more widely known HFS, which occurs with more traditional chemotherapy agents such as capecitabine.1-4, 10
Although HFS and HFSR do not involve life-threatening toxicities, the syndromes have a significant impact on treatment schedules and quality of life in treated patients.3,5,6 Management of HFS and HFSR is geared towards treating symptoms effectively and preventing them from becoming progressive and debilitating.
CLINICAL MANIFESTATIONS
Hand-foot syndrome is a potentially dose-limiting cutaneous toxicity. It is characterized by paresthesia in a sock-and-glove distribution, with varying degrees of pain, tingling, dryness, erythema, scaling, swelling, and vesiculation of the hands and feet.2,8,9 In most instances, symptoms appear after prolonged drug exposure.
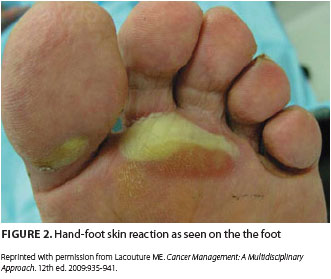 Before the onset of visible symptoms, patients usually experience dysthesia or paresthesia in the palms and soles, including numbness or tingling, which progresses over several days to a burning pain.2,3,6,8-10 Erythema and swelling develop progressively and symmetrically over these areas as well, particularly on the pads of the distal phalanges. Presenting symptoms such as these can interfere with normal activities of daily living. Blisters are more common in areas of great pressure and remain limited to the palms and, less frequently, the soles. HFSR due to TKIs usually manifests as a mild to moderate cutaneous reaction on the hands with a variant pattern, including a form in which bands of erythema alternate with normal skin.2,10
Before the onset of visible symptoms, patients usually experience dysthesia or paresthesia in the palms and soles, including numbness or tingling, which progresses over several days to a burning pain.2,3,6,8-10 Erythema and swelling develop progressively and symmetrically over these areas as well, particularly on the pads of the distal phalanges. Presenting symptoms such as these can interfere with normal activities of daily living. Blisters are more common in areas of great pressure and remain limited to the palms and, less frequently, the soles. HFSR due to TKIs usually manifests as a mild to moderate cutaneous reaction on the hands with a variant pattern, including a form in which bands of erythema alternate with normal skin.2,10
Bullous forms of HFS are more severe and tend to be associated with specific chemotherapy agents such as cisplatin (Platinol-AQ), methotrexate (Trexall), and the TKIs.2,3,5 Some studies have reported that HFSR secondary to TKIs is more likely to manifest as localized patches not only on pressure-bearing aspects of the palms and soles but also on areas that rub against neighboring surfaces, such as the lateral soles and web spaces. HFSR from TKIs can appear within the first 2 to 4 weeks of therapy,2,10 and this occurrence constitutes a variation of HFS distinct from that seen in traditional cytotoxic agents.
PATHOPHYSIOLOGY
Hand-foot syndrome was originally described in 1974 in a patient receiving mitotane (Lysodren).3,4 The syndrome was defined again in a 1982 case of a patient receiving a cytarabine-containing chemotherapy regimen and also in 1984 by Lokich and Moore in a phase I study of protracted infusion of fluorouracil. Lokich and Moore described development of a syndrome referred to as palmar-plantar erythrodysesthesia in 40% or more of the patients. The pathogenesis of HFS is poorly understood, but development of the syndrome appears to be drug-dependent and dose-dependent, with peak plasma drug concentrations, total cumulative dose, and administration schedule affecting the onset and severity.3-5 HFS symptoms may evolve as early as 24 hours after treatment initiation and as late as 10 months after continued therapy.2,3 HFS occurs when small amounts of chemotherapy drug leak out of the capillaries into the hands and feet.11 Once this leak occurs, the drug damages the surrounding tissues.
Previous studies of this syndrome suggest that histologically, the acute erythema of HFS is nonspecific and consistent with a generalized toxicity. Patterns of epidermal cytotoxic injury with mild lymphocyte-predominant dermal infiltrates are described.2,3 Three histopathologic features predominate and are typically associated with traditional chemotherapy-associated HFS and TKI-associated HFSR: dyskeratotic keratinocytes at various stages of necrosis, basal layer vacuolar degeneration, and a mild perivascular or lichenoid lymphocyte-predominant infiltrate.2,9 These histopathologic features of HFS are believed to occur through two mechanisms of action; one is direct toxic effect due to drug exposure, and the other, a new theory unique to the TKIs, suggests that inhibition of both platelet-derived growth factor receptor (PDGFR) and vascular endothelial growth factor receptor (VEGFR) in concert may impede vascular repair mechanisms.2
Direct toxicity The most commonly accepted theory of HFS pathogenesis involves direct toxicity of chemotherapeutic agents against acral epithelium.2,3,6 The most common histopathologic pattern observed—a cell-poor lymphocytic interface dermatitis with basilar vacuolar degeneration, dyskeratosis, and inflammatory changes such as dilated blood vessels, edema, and WBC infiltration—is strongly descriptive of direct toxic mechanisms of injury. Local delivery of high drug concentrations through eccrine (sweat) glands has been implicated in the etiology of HFS induced by chemotherapeutic agents such as doxorubicin and TKIs such as sorafenib.6 Notably, the palms and the soles have the highest concentration of eccrine glands in the body, thus supporting the possibility that drug accumulation in sweat results in particularly high concentrations in acral skin. One study that tested five skin surface areas of a single patient before and after doxorubicin administration demonstrated high levels of drug accumulation in the palms and soles only, and deep within the sweat duct on the palms only, supporting the theory that the drug accumulates differently in the acral areas than in the rest of the body.2,3,6
PDGFR and VEGFR inhibition The association of TKIs with the development of HFSR provides very little evidence to support a direct drug toxicity effect on the eccrine secretion properties of acral surfaces.2 The impediment of vascular repair mechanisms imposed by these agents is believed to play a significant role in the development of HFSR. Deficits of this caliber would be most clinically apparent in areas exposed to high pressure and repeat trauma, such as the palms and soles, where vessels are more likely to be damaged and dependent on intact vascular repair mechanisms.2,3,6,10
CLINICAL ASSESSMENT AND DIAGNOSIS
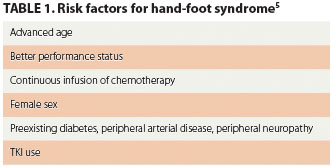 The diagnosis of HFS and HFSR is based on clinical presentation at baseline and after the initiation of therapy. Patients being treated with cytotoxic agents or TKIs that have the potential to induce cutaneous symptoms should be evaluated for such toxicities each time they are examined by a clinician. The assessment should include measures that identify and stratify patients who are at an increased risk for developing HFS and/or HFSR (Table 1).5 The clinical signs and symptoms are distinctive, and diagnosis is based on the presenting symptoms and treatment history.3 Early identification, addressing symptoms, and taking measures to prevent symptoms from worsening are imperative to improving clinical outcomes.
The diagnosis of HFS and HFSR is based on clinical presentation at baseline and after the initiation of therapy. Patients being treated with cytotoxic agents or TKIs that have the potential to induce cutaneous symptoms should be evaluated for such toxicities each time they are examined by a clinician. The assessment should include measures that identify and stratify patients who are at an increased risk for developing HFS and/or HFSR (Table 1).5 The clinical signs and symptoms are distinctive, and diagnosis is based on the presenting symptoms and treatment history.3 Early identification, addressing symptoms, and taking measures to prevent symptoms from worsening are imperative to improving clinical outcomes.
The grading system for HFS and HFSR reflects that increasing duration of exposure to the chemotherapeutic agent or TKI increases the severity of the syndrome.4,8-10 Assessment of symptoms should be graded according to appropriate staging, such as those presented in the criteria listed by the National Cancer Institute’s Common Toxicity Criteria, version 4.0. Evidence-based approaches for the prevention and treatment of HFS and HFSR support grading these symptoms to help provide the most effective and appropriate intervention.
PREVENTION AND TREATMENT
The current treatments for hand-foot symptoms are mainly anecdotal. No standard therapies have demonstrated 100% efficacy, but symptom management and evidence-based guidelines have proved to be beneficial in treating patients with HFS and HFSR. Management focuses overall on reducing modifiable risk factors that can worsen symptoms and on preventing or treating symptoms in a timely and effective manner to minimize associated physical and psychosocial discomfort and to ensure uninterrupted antineoplastic therapy.1
Clinicians must become educated about hand-foot syndromes and their implications. In turn, clinicians must educate patients undergoing treatment with cytotoxic agents or TKIs about how to recognize symptoms, along with the importance of notifying health care providers immediately at the first appearance of symptoms.7,10
PREVENTION
According to the American Society of Clinical Oncology (ASCO), patients at risk for HFS and HFSR should be instructed on the following preventive measures:
- Limiting exposure of hands and feet to hot water when washing dishes or bathing
- Taking cool showers or baths
- Avoiding exposure to sources of heat, including saunas, sitting in the sun, or sitting in front of a sunny window
- Avoiding activities that cause unnecessary force or friction on the feet, such as jogging, aerobics, and long walks
- Avoiding contact with harsh chemicals used in laundry detergents or household cleaning products
- Avoiding the use of rubber gloves to clean with hot water, as rubber traps heat against the skin
- Avoiding the use of tools or household items that require pressing the hand against a hard surface, such as garden tools, knives, and screwdrivers.7,11
- A “3C” approach to TKI-associated HFSR outlines the following guidelines for prevention:
- Controlling for calluses: before and during treatment, prophylactic removal of hyperkeratotic areas with a manicure or pedicure
- Comfort with cushions: protection of tender areas, pressure points, and pressure-sensitive areas of the hands and feet through the wearing of well-padded, well-fitted, soft shoes; foam-type absorbing soles, and shock absorbers to relieve painful pressure points
- Cover with creams: use of an emollient or keratolytic agent on callused areas of the palms and soles to moisturize and aid in natural exfoliation.10
TREATMENT
If HFS occurs despite efforts to prevent it, the focus should switch to managing existing symptoms, reducing complications, and preventing symptoms from worsening. Interventions should concentrate on lifestyle changes that avoid aggravation to the hands and feet.1-3,5,7,10 Therapies include dose modification, pyridoxine, regional cooling, celecoxib (Celebrex), topical urea, and oral corticosteroids.
Dose reduction Of all the recommended therapies for treatment of HFS, the most definitive therapy is dose reduction and treatment interruption.2,3,7 Studies show that within 2 to 4 weeks of drug cessation, HFS symptoms resolve. Manufacturer recommendations for TKIs such as sorafenib and sunitinib are to interrupt therapy for any grade 3 HFSR or for any persistent grade 2 HFSR.2 Therapy can resume after the HFSR reaches grade 0 or 1 but at a reduced dosage (sorafenib, 400 mg twice daily reduced to once daily; sunitinib, dose decreases in decrements of 12.5 mg).
Pyridoxine Very few data support the use and efficacy of vitamin B6 in managing the HFSR induced by TKIs. However, many case reports have demonstrated the beneficial effects of pyridoxine in dosages of 50 to 300 mg daily for management of HFS induced by traditional chemotherapeutic agents.2,3,7 Controlled studies have shown that the concomitant administration of pyridoxine delayed onset and reduced the severity of cutaneous toxicities associated with select chemotherapeutic agents (eg, pegylated liposomal doxorubicin).3 A small study (56 patients) reported at the ASCO 2010 Gastrointestinal Cancers Symposium found that among patients being treated with capecitabine, those taking 400 mg of pyridoxine daily had less palmar-plantar erythrodysesthesia than did patients taking 200 mg of pyridoxine daily, suggesting that the higher dose of pyridoxine was the more effective preventive therapy.12 Ongoing clinical trials hope to gain insight into the full benefit of pyridoxine in the clinical setting with regard to managing HFS and its role in HFSR induced by TKIs.
Cyclo-oxygenase-2 inhibitors Retrospective studies have demonstrated that cyclo-oxygenase-2 (COX-2) may help to mediate the inflammatory process that occurs with HFS caused by chemotherapy agents. These studies demonstrated a decreased incidence of HFS in patients treated with capecitabine.2,3 There is limited evidence to support the benefit of COX-2 agents in managing HFSR caused by TKIs.
Vasoconstrictive therapies Modalities such as localized cooling of acral areas to induce vasoconstriction can decrease the amount of drug delivered to these areas.2,3,7
Topical emollients Use of topical emollients, especially those containing lanolin, has proven to be effective in soothing affected areas of skin in HFS or HFSR. The greatest benefit of topical emollients lies in their ability to enhance moisture retention and maintain hydration, thereby reducing further desquamation and decreasing infection risks.10
CONCLUSION
Hand-foot syndromes can impair physical and psychosocial coping in patients undergoing treatment for cancer. Because these disorders can be quite debilitating and greatly compromise quality of life, management must be aggressive and timely. Early intervention is essential to preventing high grades of cutaneous toxicity and consequent treatment disruption. Care and management of HFS must also be multidisciplinary in its approach, and the role of various supportive treatment modalities continues to be investigated in the clinical arena and in clinical trials. Most importantly, educating patients about HFS and HFSR is imperative so that symptoms can be recognized early and toxicities limited. As TKIs continue to be used along with traditional chemotherapy and as new treatments continue to be developed, gaining a better understanding of the pathogenesis of hand-foot syndromes is essential. Effective management of these syndromes is imperative to improve clinical outcomes and preserve the quality of life in our patients. ONA
Jia Conway is an oncology nurse practitioner at Cancer Care Associates of York in York, Pennsylvania, and a member of the Oncology Nurse Advisor editorial board.
REFERENCES
1. Agha R, Kinahan K, Bennett CL, Lacouture ME. Dermatologic challenges in cancer patients and survivors. Oncology (Williston Park). 2007;21(12):1462-1472.
2. Lipworth AD, Robert C, Zhu AX. Hand-foot syndrome (hand-foot skin reaction, palmar-plantar erythrodysesthesia): focus on sorafenib and sunitinib. Oncology. 2009;77(5):257-271.
3. Scheithauer W, Blum J. Coming to grips with hand-foot syndrome. Insights from clinical trials evaluating capecitabine. Oncology (Williston Park). 2004;18(9):1161-1184.
4. Lokich J. The three most common chemotherapy-related skin reactions. Oncology (Williston Park). 2007;21(12). CancerNetwork Web site. http://www.cancernetwork.com/display/article/10165/63103?pageNumber=1. November 1, 2007. Accessed June 10, 2010.
5. Heo YS, Chang HM, Kim TW, et al. Hand-foot syndrome in patients treated with capecitabine-containing combination chemotherapy. J ClinPharmacol. 2004;44(10):1166-1172.
6. Milano G, Etienne-Grimaldi M, Mari M, et al. Candidate mechanisms for capecitabine-related hand-foot syndrome. Br J Clin Pharmacol. 2008;66(1):88-95.
7. Pike K. Hand-foot syndrome. Oncol Nurs Forum. 2001;28(10):1519-1520.
8. Lee C K, Lynch J. Hand-foot syndrome in breast cancer patients receiving adjuvant chemotherapy. Intern Med J. 2007;37(4):281-282.
9. Bardia A, Loprinzi CL, Goetz MP. Hand-foot syndrome after dose-dense adjuvant chemotherapy for breast cancer: a case series. J Clin Oncol. 2006;24(13);e18-e19.
10. Wood LS, Lemont H, Jatoi A, et al. Practical considerations in the management of hand-foot skin reaction caused by multikinase inhibitors. Commun Oncol. 2010;7(1):23-29.
11. Hand-foot syndrome or palmar-plantar erythodysesthesia. American Society of Clinical Oncology Web site. http://www.cancer.net/patient/All+About+Cancer/Treating+Cancer/Managing+Side+Effects/Hand-Foot+Syndrome+or+Palmar-Plantar+Erythrodysesthesia. June 2009. Accessed June 10, 2010.
12. Chalermchai V, Sriuranpong V. A randomized trial of two different doses of pyridoxine in the prevention of capecitabine-associated palmar-plantar erythrodysesthesia. Abstract 468. American Society of Clinical Oncology 2010 Gastrointestinal Cancers Symposium. http://www.asco.org/ASCOv2/Meetings/Abstracts?&vmview=abst_detail_view&confID=72&abstractID=1262. Accessed June 10, 2010.
HOW TO TAKE THE POST-TEST: To obtain CE credit, please click here after reading the article to take the post-test on myCME.com.