Data on use of a 500-mg dose of fulvestrant (Faslodex) injection was presented at the Breast Cancer Symposium. Are there any policies or guidelines for giving these high-dose injections?
—Denise Smith, RN, OCN
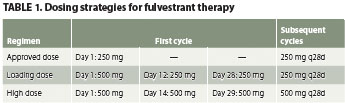
Fulvestrant is an estrogen receptor antag-onist used to treat hormone receptor-positive metastatic breast cancer in postmenopausal women whose cancer hasprogressed on first-line antiestrogen ther-apy. It is administered by IM injection into the buttock. The FDA-approved dosage (AD) is 250 mg every 28 days. Research has focused on whether alternate dosages have a faster onset of action or are more effective. Two alternate strategies are the high dose (HD) and loading dose (LD) regimens (Table 1).
Fulvestrant is supplied as a 50 mg/mL suspension in prefilled syringes. The 250-mg AD is administered using one 5-mL or two 2.5-mL IM injections (one into each buttock), depending on patient size and tolerability of injection. A 500-mg dose requires administration of a total of 10 mL, so using two 250-mg syringes is advised. The studies that investigated 500-mg LDs and HDs administered the dose as one 250-mg syringe IM into each buttock.1,2 Adverse effects that were reported in clinical trials, including injection site pain, were similar between the three dosing regimens.
The EFECT study compared the efficacy of the fulvestrant LD regimen with exemestane (Aromasin) in postmenopausal women with hormone receptor-positive advanced breast cancer. Women receiving the fulvestrant LD regimen reached steady-state levels of the drug within 28 days, compared to 3 to 6 months with the fulvestrant AD regimen, with no increase in adverse effects. This study did not compare differences in efficacy between the AD and LD regimens.1
The phase III CONFIRM study compared the AD and HD regimens of fulvestrant in postmenopausal women with hormone receptor-positive breast cancer that had progressed on first-line endocrine therapy.2 Women in the HD group experienced a longer time to progression. Overall response rate and quality of life were similar between the groups. Further analysis of safety and efficacy is ongoing.2 ONA
Lisa Thompson is Assistant Professor, Department of Clinical Pharmacy, University of Colorado Denver School of Pharmacy, Aurora, Colorado.
REFERENCES
1. Chia S, Gradishar W, Mauriac L, et al. Double-blind, randomized placebo controlled trial of fulvestrant compared with exemestane after prior nonsteroidal aromatase inhibitor therapy in postmenopausal women with hormone receptor-positive, advanced breast cancer: results from EFECT. J Clin Oncol. 2008;26(10):1664-1670.
2. Di Leo A, Jerusalem G, Petruzelka L, et al; the CONFIRM Investigators. CONFIRM: a phase III, randomized, parallel-group trial comparing fulvestrant 250 mg vs fulvestrant 500 mg in postmenopausal women with estrogen receptor-positive advanced breast cancer. In: San Antonio Breast Cancer Symposium. San Antonio, TX; CTRC-AACR; 2009. Abstract 25.
