8 Sengkang food stalls that you must try

The Sengkang food scene has been picking up lately, with recent news of a brand new Sengkang hawker centre slated for early 2024.
Fernvale Hawker Centre & Market opened in September last year, too.
In the meantime, here’s HungryGoWhere’s top eight hawker picks for Sengkang food, whether you’re simply ravenous or just looking for a light nibble.
1. Wonton Mama
FoodHub Coffee Shop, 01-01, 455 Sengkang West Avenue
Open: Tuesdays (8am to 12am), Wednesdays to Saturdays (12am to 12am), Sundays (12am to 6pm)

Why visit? This coffee-shop stall at Sengkang West Avenue is a good bet for Thai food in Sengkang if you’re feeling the late night munchies. It’s usually open until late (and even around the clock for most of the week).
Opened since 2021, it serves a range of Thai dishes, including seafood tom yum (S$10.90). There’s also a toothsome grilled pork collar (S$8.50) that’s been marinated for at least 24 hours before being grilled.
Its housemade fried chilli promises quite the spicy kick, too.
Fun fact: This is the sister brand of Tomyum Mama in Upper Thomson, which in turn is known for its signature claypot tom yum with Mama instant noodles.
Price range: $$
Crowd faves: It is best known for its Thai-style wanton noodles (S$9.50), which come with dumplings, fish sausage slices and even a ramen egg with a gooey centre.
2. Geetham Restaurant
Kopitiam Food Court, 01-52, 10 Sengkang Square
Open: Mondays to Sundays (12am to 12am)

Why visit? Located in Sengkang Square, this 24-hour Indian-Muslim stall prides itself on not using MSG and re-used oil in its dishes.
You’ll find the usual suspects and more on the menu: Everything from snacks such as idiyappam — or putu mayam — (S$1, rice flour pancake) to biryani, nasi goreng and other carbolicious bombs.
Price range: $$
Crowd faves: Get a power-packed nasi biryani (from S$7), which comes with a wide choice of side dishes, including mutton, prawns, chicken and fish.
3. Jun Yuan House of Fish
Kopitiam Food Court, 01-59, 10 Sengkang Square
Open: Sundays to Mondays (10am to 8.30pm)

Why visit? A Michelin Bib Gourmand recipient for the years 2021 and 2022, Jun Yuan House of Fish has grown far beyond its original Old Airport Road stall. The chain now boasts five stores islandwide, with its latest Roxy Square outpost opening in February 2023.
As its name will tell you, the stall serves sliced fish soup in several variations — a perennial favourite among Singaporean office-goers.
Price range: $
Crowd faves: Its owners consider the herbal seafood soup (from S$5) their signature. The broth is richly laden with a melange of herbs, and diners can choose from various types of fish, including dory, batang and black grouper.
4. Munchi Pancakes
Fernvale Hawker Centre & Market, 03-04, 21 Sengkang West Avenue
Open: Mondays to Sundays (8am to 8.30pm)

Why visit? Feeling snacky? Munchi Pancakes at Fernvale serves up min jiang kueh (peanut pancakes) with a modern twist. Not only will you find the snack in its traditional form and fillings, you’d also get it in new flavours such as charcoal and matcha.
What draws many customers are its Insta-worthy colours, which depend on your chosen flavour. The charcoal pancakes of course take on a dark hue, while the matcha pancakes are tinged green.
Munchi has also created modern pancakes, which are round and enclosed.
The stall is halal, too.
Price range: $
Crowd faves: The original min jiang kueh (S$1.50) is a solid choice, but you should definitely try some of the more unique flavours, including biscoff min jiang kueh (S$1.90). Try the stall’s modern Munchi pancakes too, which come in 12 flavours, including strawberry cheese (S$1.90), Thai milk tea (S$1.90) and cream cheese (S$1.90).
5. Feng Xiang Herbal Bak Kut Teh
Fernvale Hawker Centre & Market, 03-01, 21 Sengkang West Avenue
Open: Mondays to Fridays (10am to 3pm, 4.30pm to 8.30pm), Saturdays and Sundays (9.30am to 3pm, 4.30pm to 8.30pm)

Why visit? The folks behind Feng Xiang Herbal Bak Kut Teh have had a busy couple of years. Since 2021, they’ve opened five stores at various locations.
Harvey Ang, its founder, started the business at the height of the pandemic and found loyal customers among diners who missed the taste of good, authentic Malaysian recipes.
Price range: $$
Crowd faves: Feng Xiang’s herbal bak kut teh (from S$6.90, pork ribs tea soup) is stewed for many hours to bring out the flavour of its herbs and pork bones, and is a must-try. Diners also love its fried porridge (from S$6.90) — a recipe that originates from Klang, Malaysia — that involves frying the dish in a wok to infuse it with smoky wok hei (breath of the wok).
6. Roger’s Kitchen
Aspella, 01-01, 275D Compassvale Link
Open: Mondays to Sundays (11am to 10pm)
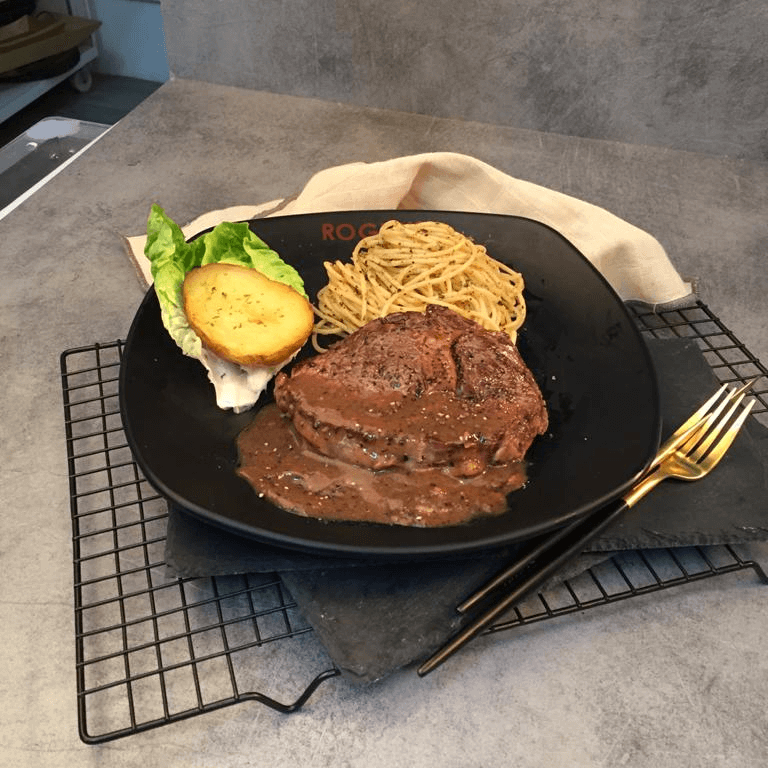
Why visit? This unassuming stall tucked away in a housing development block is known for its generous portions of Western hawker fare at affordable prices, and a go-to for good food in Sengkang. There are premium options too, including lamb chop, beer-battered fish and 200-day grass-fed ribeye steaks.
Price range: $$
Crowd faves: Diners love its creamy truffle carbonara with fried butter chicken (S$8.50) and grilled chicken chop with pasta (S$8). If you’re feeling adventurous, check out the mala pasta with fried butter chicken (S$7.90).
7. Xing Le Mala Hotpot
Happy Hawkers, 01-02, 267 Compassvale Link
Open: Mondays to Sundays (12.30pm to 12am)

Why visit? Craving mala? Xing Le Mala Hotpot’s got your back. Besides the typical spread of mala xiang guo (stir fried hotpot) ingredients, you can also take your pick from various zi char (cook and fry) dishes and sides.
Price range: $$$
Crowd faves: You just can’t visit a mala store without trying its mala xiang guo. As usual, prices vary depending on your choice of ingredients — these start from S$1.30 for vegetables and carbs, and S$3 for meats.
8. Lin Yu Mei Kolo Mee
Kwek Seng Huat Eating House, 01-01, 350A Anchorvale Road
Open: Wednesdays to Sundays (8am to 6pm)

Why visit? Lin Yu Mei’s northeastern outpost sees brisk traffic and regular customers who come back for its authentic kolo mee and Sarawak laksa recipes.
The laksa sold here is aromatic and flavourful, but markedly different compared to the Katong laksa that many Singaporeans are used to. It’s less curry-like, and more tangy.
Price range: $
Crowd faves: Its dry kolo mee dry($6.20) boasts springy, QQ noodles, slices of chilli, and best of all, housefried chilli. The Sarawak laksa (S$9.50) too, is a popular order.
Looking for more affordable eats? Check out our guide on meals under S$10 in the CBD. Otherwise, find out Member of Parliament Jamus Lim’s go-to food haunts in Sengkang.
All the stalls on this list, except Munchi Pancake, are on the GrabFood delivery service and offer free delivery (up to S$3 off) with GrabUnlimited.
Alternatively, book a ride to these Sengkang food spots.







