| The following article features coverage from the 2020 ONA Virtual Navigation Summit. Click here to read more of Oncology Nurse Advisor‘s conference coverage. In addition, the original presentation is available for on-demand viewing and CNE credit until September 2021, click here to access. |
Patients undergoing chimeric antigen receptor T-cell (CAR-T) therapy will experience challenges and potential barriers unique to this treatment. In this presentation, we offer solutions to help navigate patients through and around these barriers, and discuss posttreatment and long-term survivorship care.
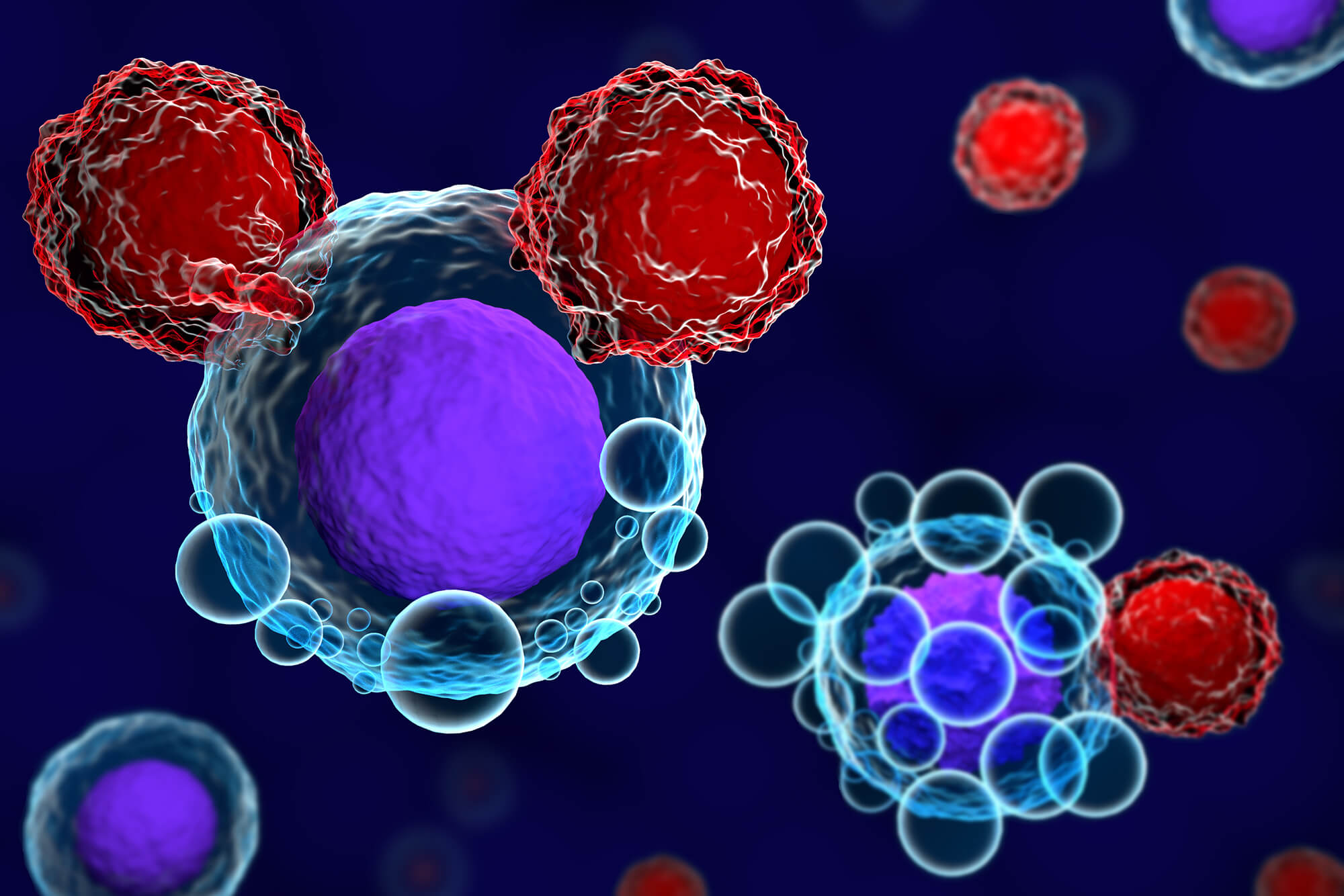
T lymphocytes (T-cells) are the “soldiers” of the immune system. They can kill abnormal cells by recognizing peptides presented in HLA molecules. Adoptive T-cell therapies involve engineering T-cells to recognize and attack tumor cells. CAR-T cells are engineered to express a synthetic T-cell receptor for a tumor.
CAR-T therapy is a 4-step process. First, T-cells are harvested from the patient’s blood. In the laboratory, the T-cells are engineered to become CAR T-cells, then allowed to multiply until there are millions of them. The last step is to return the now-engineered T-cells to the patient’s bloodstream where they recognize and kill cancer cells.
Currently, 4 agents have been granted FDA approval: Tisagenlecleucel (Kymriah®) is approved for the treatment of patients younger than 26 years with relapsed/refractory (r/r) acute lymphocytic leukemia and for the treatment of adults with r/r large B-cell lymphoma after 2 or more lines of systemic therapy. Axicabtagene ciloleucel (Yescarta®) is approved for the treatment of adults with r/r large B-cell lymphoma after 2 or more lines of systemic therapy; and brexucabtagene autoleucel (Tecartus™) was recently approved for the treatment of adults with r/r Mantle cell lymphoma.
Other CAR-T therapies currently in development are lisocabtagene maraleucel, a CD19 CAR-T, and idecabtagene vicleucel, orvacabtagene autoleucel, and JNJ-4528, which are BCMA CAR-T therapies.
Common toxicities of therapy are tumor lysis syndrome, B cell aplasia, hemophagocytic lymphohistiocytosis (HLH)-like syndrome, disseminated intravascular coagulation (DIC), cytopenias, and infection. However, the 2 major treatment-related complications are cytokine release syndrome (CRS) and neurotoxicity, which is now formally called immune effector cell-associated neurotoxicity syndrome (ICANS).
Overall, the general complications of CRS and ICANS appear to be similar across the FDA-approved agents, but their frequency, rate of onset, and general peak level of toxicity varies. Regarding efficacy, the response rates for each of the 3 approved therapies are comparable.
The barriers unique to CAR-T therapy are geographical factors (must be within 30-120 minutes travel time of the treatment facility), length of treatment (approximately 2 months), financial burden (travel and lodging costs, as well as medical expenses), caregiver requirements, and high-risk population. Some strategies navigators can use to help patients navigate through this often difficult process include early initiation of financial clearance, clear and thorough communication with the patient and their caregivers, coordination with internal patient resources, awareness of community resources available to this population of patients, and access to patient-learning resources.
Patients and their caregivers must remain close to the treatment facility for 1 month after CAR T-cell infusion. After that time, the patient is transitioned back to their oncologist for continued follow-up care. The FDA recommends following patients for 15 years posttreatment.
The components of follow-up care include an annual physical examination; monitoring for replication-competent retroviruses (RCRs) and persistence every 6 months for years 1 to 5, then annually for years 5 to 15; monitoring for adverse events of special interest (AESI) related to gene therapy; revaccination; and quality of life questionnaires.