Navigating the Complexity of EGFR Exon 20 Insertion Mutations in NSCLC: Clinical Implications and Therapeutic Challenges
Non-small cell lung cancer (NSCLC) accounts for 85% of lung cancer cases worldwide and remains a significant contributor to cancer-related mortality.1 Late-stage diagnoses contribute to the poor long-term survival in patients with lung cancer, as 57% of patients present with metastatic lung cancer upon detection.2
NSCLC is characterized by heterogeneity and encompasses various histological subtypes, such as adenocarcinoma, squamous cell carcinoma, and large cell carcinoma, among others.3 The diverse genetic mutations within NSCLC significantly impact treatment strategies, emphasizing the critical role of molecular testing and targeted therapies. Molecular testing plays a pivotal role in treatment decisions, especially concerning targeted therapies aimed at specific oncogenic mutations within NSCLC.4
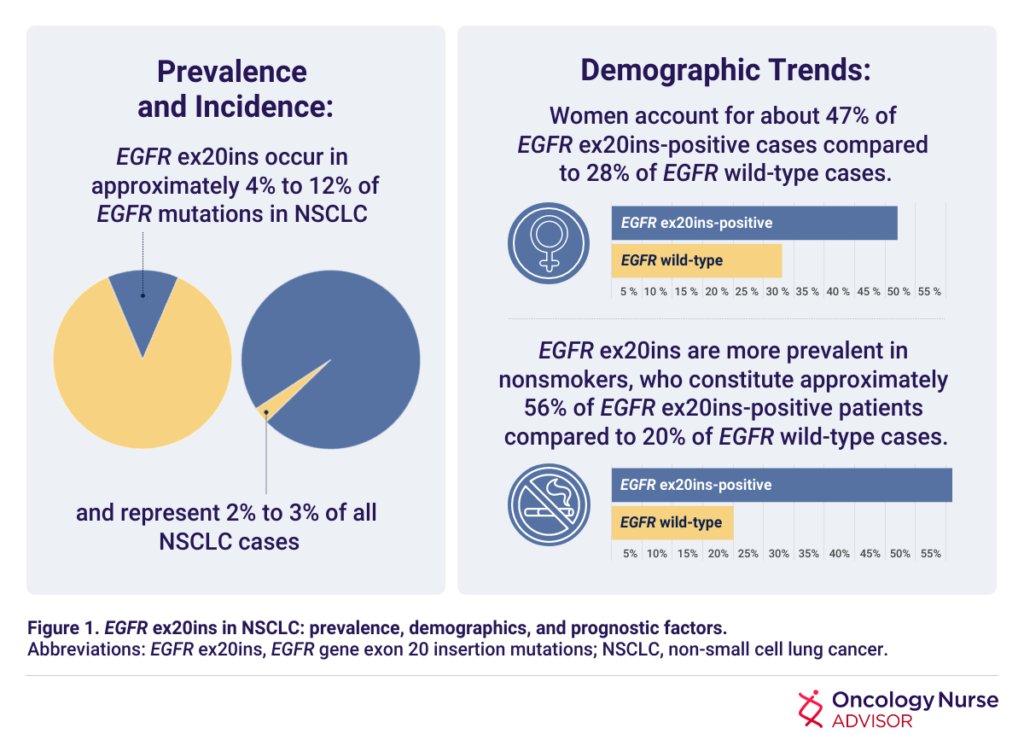
Despite the significant strides made in personalized treatments in recent years, NSCLC continues to pose formidable challenges in clinical management and outcomes. Metastatic NSCLC, in particular, remains a challenging and often fatal disease, marked by a 5-year survival rate of less than 10%. Exon 20 insertion (ex20ins) mutations in the epidermal growth factor receptor (EGFR) gene have been identified as oncogenic drivers in NSCLC, and patients with NSCLC with EGFR ex20ins mutations do not respond well to treatment with EGFR tyrosine kinase inhibitors (TKIs).5 The prevalence, incidence, and demographic trends of EGFR ex20ins mutations in NSCLC are listed in Figure 1.5-7
Molecular and Clinical Characteristics
EGFR ex20ins mutations demonstrate heightened EGFR kinase activity and exhibit structural differences from well-characterized mutations, such as exon 19 deletions and L858R missense mutations. Unlike these known mutations, EGFR ex20ins mutations — with over 60 variants in length and sequence — present challenges in responsiveness to therapeutic interventions.8
Clinically, patients with EGFR ex20ins-positive NSCLC tend to be younger, with adenocarcinoma emerging as the predominant subtype associated with these mutations. Patients with EGFR ex20ins display a notably poorer prognosis compared with those with classical EGFR mutations, with distinct metastatic patterns predominantly involving bone, central nervous system, and liver.7
Diagnostic Testing
Diagnostic testing plays a crucial role in diagnosing NSCLC, particularly in detecting pivotal mutations such as EGFR mutations. Methods such as liquid biopsy or tumor tissue sequencing are used to identify EGFR mutations, as EGFR mutations generally consist of point mutations or variably sized mutations below the threshold of testing for karyotype, fluorescence in situ hybridization (FISH), or immunohistochemistry (IHC) staining. Tissue analysis by next-generation sequencing (NGS) is considered the gold standard of diagnosis for this condition due to its accurate detection of multiple gene aberrations. However, there are notable differences in the turnaround times for testing EGFR ex20ins.4
One study found that the median time from diagnosis to the identification of a patient’s EGFR exon20ins was 28 days for NGS, whereas polymerase chain reaction (PCR) methods have an average turnaround time of approximately 12 days,4 underscoring the pressing need for more efficient and expedited diagnostic processes focused on identifying these specific mutations.
Understanding the Challenges of EGFR ex20ins Mutations in Responding to Standard Therapies
Although EGFR TKIs are the first choice of therapy for most patients with NSCLC with EGFR mutations, their lack of efficacy for patients with EGFR ex20ins mutations lead to the continued use of platinum-based chemotherapy as the first-line treatment for EGFR ex20ins mutations in most contexts. In 2 studies conducted in China, individuals with EGFR ex20ins mutations who were treated with platinum-based chemotherapy as a first-line treatment exhibited an objective response rate (ORR) of 19% and 19.2% and a median progression-free survival (PFS) of 6.4 and 6.5 months.1 Another study performed in China noted that pemetrexed-based chemotherapy provides potentially superior disease control compared with non-pemetrexed regimens, offering an improved PFS of 5.5 vs 3.0 months.4
Immune checkpoint inhibitors (ICIs) do not confer significant benefits for individuals with EGFR mutations. However, limited research shows that patients with EGFR ex20ins mutations receive more benefits from ICIs than do patients with other types of EGFR mutations. In 1 prospective study, patients with EGFR ex20ins mutations who received ICI therapy demonstrated an ORR of 25% and a PFS of 2.5 months, whereas patients with classical EGFR mutations demonstrated an ORR of 0% and a PFS of 1.9 months.4 However, further studies are needed to more fully understand the impact of ICI treatment in patients with EGFR ex20ins mutations.
Mobocertinib
In 2021, mobocertinib was approved by the US Food and Drug Administration (FDA) for adult patients with NSCLC and EGFR ex20ins mutations whose disease progressed on or after platinum-based chemotherapy.9 Mobocertinib is an oral TKI that selectively targets EGFR and human epidermal growth factor receptor 2 (HER2) ex20ins.3 Clinical data from the phase 1/2 EXCLAIM (ClinicalTrials.gov identifier: NCT02716116) multicenter study found that patients with NSCLC and EGFR ex20ins mutations who previously were treated with 1 or 2 lines of therapy demonstrated an ORR of 43% and a PFS of 7.3 months when treated with mobocertinib. The most common treatment-related adverse events (AEs) observed with mobocertinib were diarrhea and rash; approximately 22% to 25% of patients developed AEs that led to dose modification, and 10% to 17% of patients discontinued treatment due to AEs.10
Amivantamab
Amivantamab was FDA-approved in 2021 for adult patients with NSCLC and EGFR ex20ins mutations whose disease progressed on or after platinum-based chemotherapy.11 Amivantamab is a bispecific antibody with a unique mechanism that involves binding to EGFR and mesenchymal-epithelial transition (MET) receptors, effectively inhibiting ligand-induced phosphorylation.8
Amivantamab has shown impressive results; in the CHRYSALIS trial (ClinicalTrials.gov identifier: NCT02609776) patients with NSCLC and EGFR ex20ins mutations who were previously treated with platinum-based chemotherapy demonstrated an ORR of 40% and a PFS of 8.3 months. The most common AEs seen with amivantamab included rash, paronychia, stomatitis, pruritus, hypoalbuminemia, peripheral edema, and diarrhea.12
Further details on FDA-approved targeted therapies for the treatment of patients with NSCLC and EGFR ex20ins mutations are listed in Table 1.13,14

Ongoing Research and Future Prospects
Continued trials and postmarketing commitments aim to delve deeper into clinical benefits, safety, and real-world impact of both mobocertinib and amivantamab.
As a follow up to the EXCLAIM trial, EXCLAIM-2 (ClinicalTrials.gov identifier: NCT04129502) is investigating the comparative efficacy of mobocertinib vs platinum-based chemotherapy as a first-line therapy for patients with NSCLC and EGFR ex20ins mutations, with an estimated completion date in March 2025.
The CHRYSALIS trial is also ongoing; once it is completed, it is expected to provide a greater scope of data regarding the safety, pharmacokinetics, and preliminary efficacy of amivantamab as both a monotherapy and in combination with lazertinib for patients with advanced NSCLC.
References
1. Hou J, Li H, Ma S, et al. EGFR exon 20 insertion mutations in advanced non-small-cell lung cancer: current status and perspectives. Biomark Res. 2022;10(1):21. doi:10.1186/s40364-022-00372-6
2. Russell MC, Garelli AM, Reeves DJ. Targeting EGFR exon 20 insertion mutation in non-small cell lung cancer: amivantamab and mobocertinib. Ann Pharmacother. 2023;57(2):198-206. doi:10.1177/10600280221098398
3. Arnold A, Ganti AK. Clinical utility of mobocertinib in the treatment of NSCLC – patient selection and reported outcomes. Onco Targets Ther. 2023;16:559-569. doi:10.2147/OTT.S374489
4. Brazel D, Kroening G, Nagasaka M. Non-small cell lung cancer with EGFR or HER2 exon 20 insertion mutations: diagnosis and treatment options. BioDrugs. 2022;36(6):717-729. doi:10.1007/s40259-022-00556-4
5. Chon K, Larkins E, Chatterjee S, et al. FDA approval summary: amivantamab for the treatment of patients with non-small cell lung cancer with EGFR exon 20 insertion mutations. Clin Cancer Res. 2023;29(17):3262-3266. doi:10.1158/1078-0432.CCR-22-3713
6. Ou SI, Lin HM, Hong JL, et al. Real-world response and outcomes in patients with NSCLC with EGFR exon 20 insertion mutations. JTO Clin Res Rep. 2023;4(10):100558. doi:10.1016/j.jtocrr.2023.100558
7. Pan B, Liang J, Shi H, Rao K, Guo W, Zhan C. Epidemiological characteristics and therapeutic advances of EGFR exon 20 insertion mutations in non-small cell lung cancer. Thorac Cancer. 2023;14(33):3247-3258. doi:10.1111/1759-7714.15127
8. Petrini I, Giaccone G. Amivantamab in the treatment of metastatic NSCLC: patient selection and special considerations. Onco Targets Ther. 2022;15:1197-1210. doi:10.2147/OTT.S329095
9. FDA grants accelerated approval to mobocertinib for metastatic non-small cell lung cancer. News release. US Food and Drug Administration. September 16, 2021. Accessed December 10, 2023. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-mobocertinib-metastatic-non-small-cell-lung-cancer-egfr-exon-20
10. Bai Q, Wang J, Zhou X. EGFR exon20 insertion mutations in non-small cell lung cancer: clinical implications and recent advances in targeted therapies. Cancer Treat Rev. 2023;120:102605. doi:10.1016/j.ctrv.2023.102605
11. FDA grants accelerated approval to amivantamab-vmjw for metastatic non-small cell lung cancer. News release. US Food and Drug Administration. May 21, 2021. Accessed December 19, 2023. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-amivantamab-vmjw-metastatic-non-small-cell-lung-cancer
12. Park K, Haura EB, Leighl NB, et al. Amivantamab in EGFR exon 20 insertion-mutated non-small-cell lung cancer progressing on platinum chemotherapy: initial results from the CHRYSALIS phase I study. J Clin Oncol. 2021;39(30):3391-3402. doi:10.1200/JCO.21.00662
13. ExkivityTM. Prescribing information. Takeda Pharmaceuticals America, Inc; 2023. Accessed December 10, 2023. https://www.exkivity-update.com/prescribing-information.pdf
14. Rybrevant®. Prescribing information. Janssen Biotech, Inc; 2022. Accessed December 10, 2023. https://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/RYBREVANT-pi.pdf
Posted by Haymarket’s Clinical Content Hub. The editorial staff of Oncology Nurse Advisor had no role in this content’s preparation.
Reviewed January 2024